
EMA Grants Orphan Drug Designation to NIDO-361 for Kennedy’s Disease
Nido Biosciences, a biopharmaceutical company developing medicines to treat debilitating neurological diseases with precision, today announced that the European Medicines Agency has granted an Orphan ...
European Commission Grants Orphan Designation to Theriva Biologics’ VCN-01 for Retinoblastoma
Theriva Biologics, a clinical-stage biotech company developing cancer therapeutics, announced that the European Commission has adopted the EMA's recommendation to grant orphan medicinal product design ...
FDA Grants Rare Pediatric Disease Designation to Papillon Therapeutics’ PPL-001 for Friedreich’s Ataxia
Papillon Therapeutics Inc., a clinical-stage biotechnology company focused on developing multi-systemic genetic medicines to address the root causes of inherited diseases, has announced that the U.S. ...
FDA Grants Orphan Drug Designation to Immuneering’s IMM-1-104 for Pancreatic Cancer Treatment
Immuneering Corporation, a clinical-stage oncology company focused on developing universal RAS/RAF medicines for a broad range of cancer patients, has announced that the U.S. Food and Drug Administrat ...
CyGenica Receives Orphan Drug Designation for Glioblastoma Multiforme (GBM) Treatment
CyGenica Limited, a dynamic biotech startup, has achieved a significant milestone in the fight against Glioblastoma Multiforme (GBM), an aggressive form of brain cancer. Dr. Nusrat J M Sanghamitra, Co ...
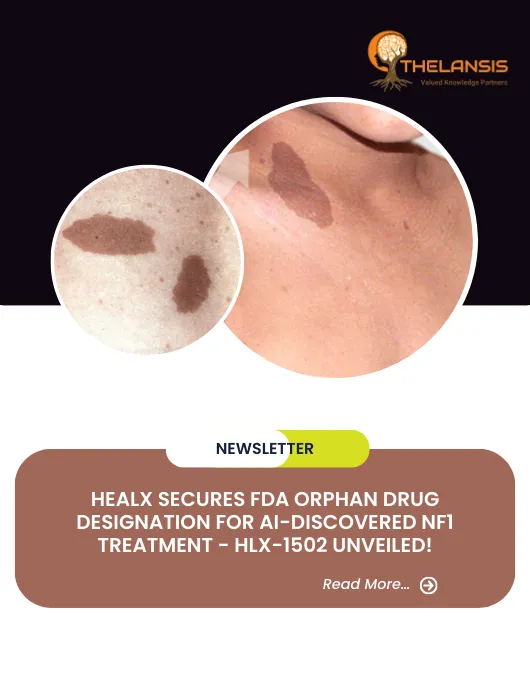
Healx Secures FDA Orphan Drug Designation for AI-Discovered NF1 Treatment – HLX-1502 Unveiled!
Healx, a cutting-edge techbio company driven by AI innovation and patient-centricity, has achieved Orphan Drug Designation from the US Food and Drug Administration (FDA) for its groundbreaking AI-driv ...
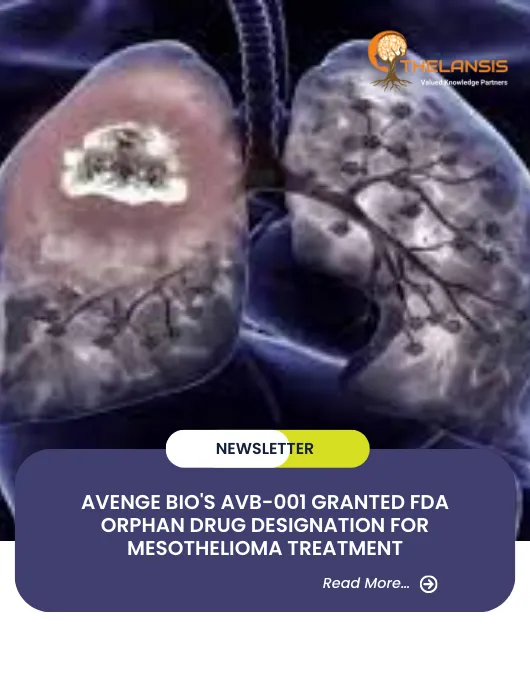
Avenge Bio’s AVB-001 Granted FDA Orphan Drug Designation for Mesothelioma Treatment
Avenge Bio, Inc., a leading biotech firm specializing in oncology, has received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for AVB-001. This designation recognizes AVB-00 ...
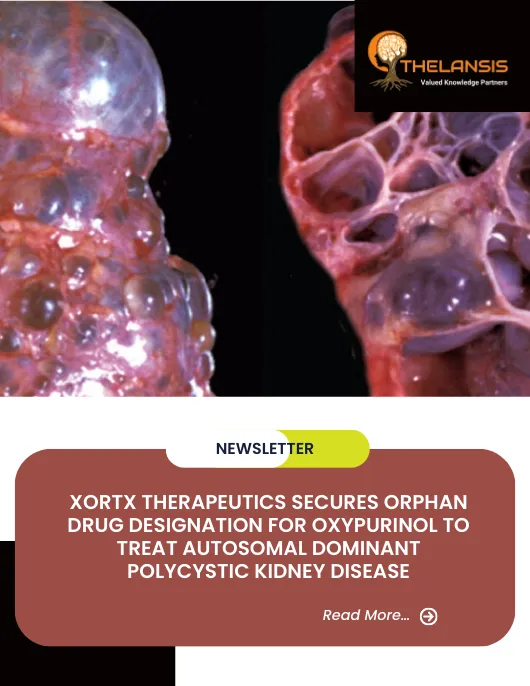
XORTX Therapeutics Secures Orphan Drug Designation for Oxypurinol to Treat Autosomal Dominant Polycystic Kidney Disease
XORTX Therapeutics Inc., a late-stage clinical pharmaceutical company that aims to create new therapies to treat progressive kidney disease, is excited to announce that they have received Orphan Drug ...
Sumitomo Pharma’s TP-1287 granted FDA Orphan Drug and Rare Pediatric Disease Designations for Ewing sarcoma
Sumitomo Pharma Oncology, Inc. has received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for TP-1287, an oral CDK9 inhibitor that is being investigated for the treatment of ...
Abbisko Cayman’s ABSK012 Granted FDA Orphan Drug Designation for Soft Tissue Sarcoma Treatment
Abbisko Therapeutics, a subsidiary of Abbisko Cayman, has been awarded an orphan drug designation by the US Food and Drug Administration (FDA) for ABSK012, an FGFR4 mutant inhibitor that treats soft t ...

