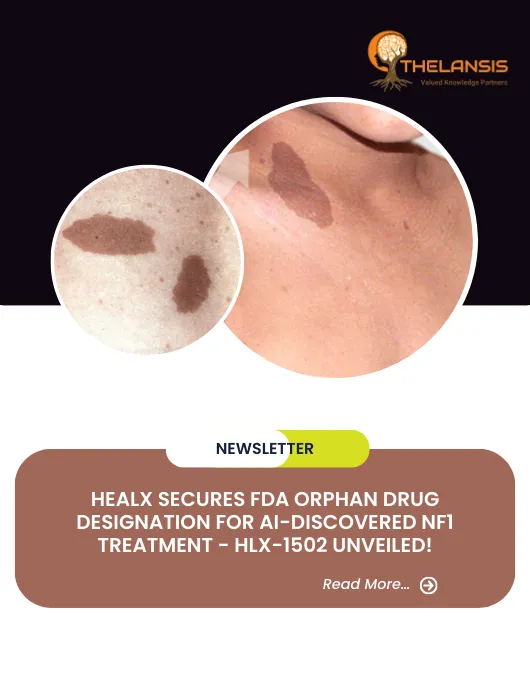
Healx Secures FDA Orphan Drug Designation for AI-Discovered NF1 Treatment - HLX-1502 Unveiled!
Healx, a cutting-edge techbio company driven by AI innovation and patient-centricity, has achieved Orphan Drug Designation from the US Food and Drug Administration (FDA) for its groundbreaking AI-driven solution targeting Neurofibromatosis Type 1 (NF1). With a dedicated team of skilled scientists and technologists, Healx harnessed the power of its pioneering AI drug discovery platform to identify HLX-1502 as a promising candidate for NF1 treatment.
Publish Date: 06-09-2023 Source: Healx
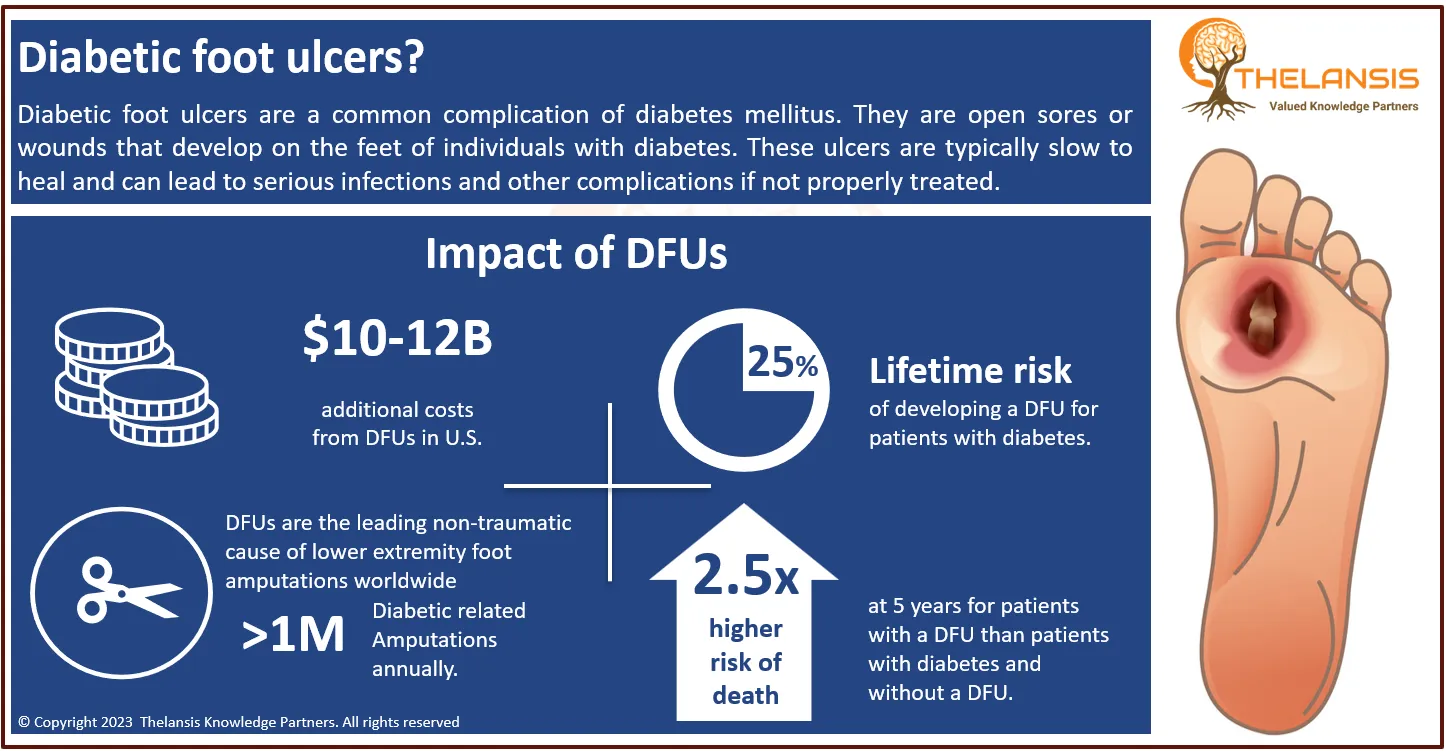
Neurofibromatosis type 1 (NF1) is a neurocutaneous genetic disorder known for its diverse clinical manifestations, including café-au-lait spots, Lisch nodules in the iris, freckling in axillary and inguinal regions, and multiple neurofibromas. NF1 condition exhibits significant variability in clinical features, even among individuals within the same family. Almost all patients display multiple café-au-lait macules, some present at birth and most emerging within the first year of life. Intertriginous freckling typically begins around the age of 5. In adults, cutaneous and subcutaneous neurofibromas develop and continue to increase in number and size with age. It’s important to note that these cutaneous neurofibromas do not turn malignant. However, plexiform neurofibromas, which grow along nerves and their branches, may cause disfigurement, pain, and functional impairments. They are usually present at birth and may become malignant later in life. Ocular manifestations of NF1 include optic pathway gliomas and iris hamartomas (Lisch nodules). Optic pathway gliomas usually appear before age 6 and rarely progress beyond that age. Additionally, NF1 can lead to various skeletal issues, such as osteopenia, osteoporosis, bone overgrowth, short stature, microcephaly, scoliosis, and various skeletal dysplasias. Other associated features encompass hypertension, vasculopathy, intracranial tumors, malignant peripheral nerve sheath tumors (MPNST), and, occasionally, seizures or hydrocephalus. While intellectual development is typically not severely affected, cognitive deficits and learning difficulties are common in 50% to 75% of cases. The overall cancer risk in NF1 is higher than in the general population, with a lifetime risk of 10-12% for MPNST, primarily occurring between ages 20 and 40, and an elevated risk of breast cancer before age 50. Variants of NF1, such as familial spinal and segmental forms and Watson syndrome, exist within the NF1 spectrum. Neurofibromatosis-Noonan syndrome represents a variant in 99% of cases. To diagnose NF1 formally, specific criteria have been established. Two or more of the following are diagnostic: more than 5 café-au-lait macules, 2 or more neurofibromas or one plexiform neurofibroma, optic glioma, freckling, 2 or more Lisch nodules, specific bone dysplasias, or a first-degree relative with NF1. Magnetic resonance imaging can assess the extent of plexiform neurofibromas, while molecular genetic testing is rarely necessary. Legius syndrome, observed in about 2% of individuals meeting NF1 diagnostic criteria, often closely resembles NF1 clinically. However, some individuals with NF1 may not develop non-pigmentary manifestations, resembling Legius syndrome patients. Constitutional mismatch repair deficiency syndrome should be considered a differential diagnosis in certain cases. Other differential diagnoses include McCune-Albright syndrome, Noonan syndrome with lentigines, and Proteus syndrome. Most instances of multiple non-ossifying fibromatosis can be attributed to NF1. The overall prognosis for NF1 is generally favorable, but significant morbidity is common. MPNST, in particular, carries a poor prognosis. Malignancy and vascular disease are the leading causes of early mortality. Currently, no treatment to reverse NF1 exists, but its signs and symptoms can be managed and addressed.
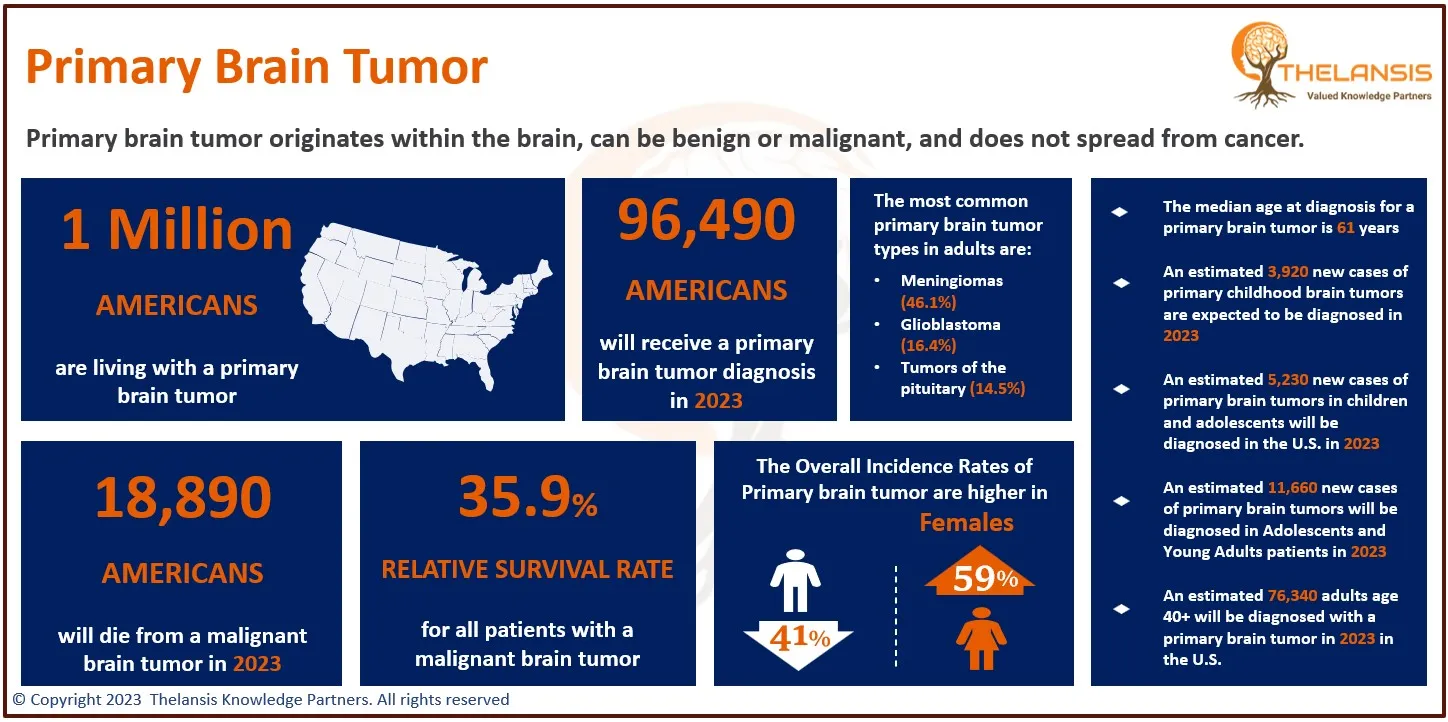
- NF1 is considered one of the most common genetic conditions, affecting an estimated 1 in 3,000 people worldwide, with up to 30% to 50% of affected individuals having no family history.
However, the current Neurofibromatosis Type 1 (NF1) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Selumetinib, REC-2282, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Neurofibromatosis Type 1 treatment. The key companies in the advanced development stage are AstraZeneca, Recursion Pharmaceuticals Inc., etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Neurofibromatosis Type 1 (NF1) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2022 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com