
AnGes’ Lonafarnib Granted Orphan Drug Designation by Japanese Ministry of Health for HGPS Treatment
Anges Inc. announced that Lonafarnib (marketed in the US as Zokinvy), a medication designed to treat Hutchinson-Gilford Progeria Syndrome (HGPS) and Processing-Deficient Progeroid Laminopathy (PL), ha ...
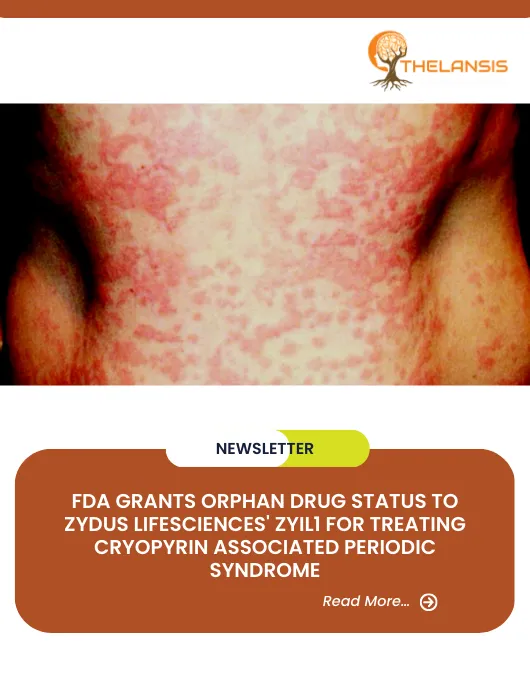
FDA Grants Orphan Drug Status to Zydus Lifesciences’ ZYIL1 for Treating Cryopyrin-Associated Periodic Syndrome
Zydus Lifesciences has announced that its drug, ZYIL1, has received orphan drug designation (ODD) from the United States Food and Drug Administration (US FDA) for the treatment of Cryopyrin Associated ...
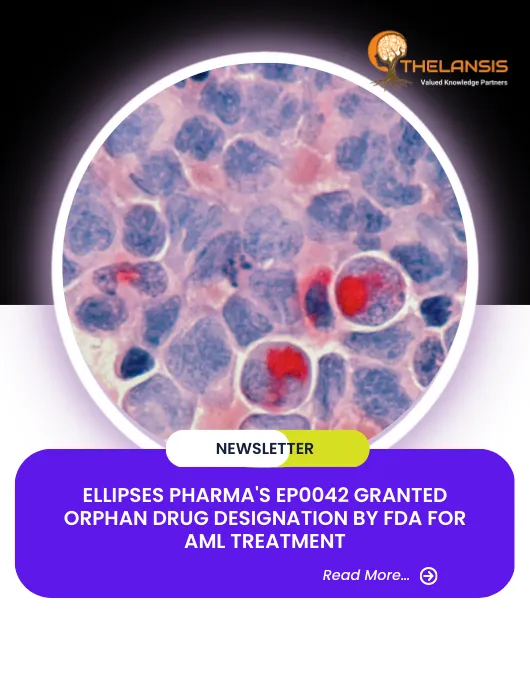
Ellipses Pharma’s EP0042 Granted Orphan Drug Designation by FDA for AML Treatment
Ellipses Pharma obtains Orphan Drug Designation (ODD) from the FDA for EP0042, a dual FLT-3 and Aurora kinase inhibitor, as a treatment for acute myeloid leukaemia (AML). This designation is granted t ...
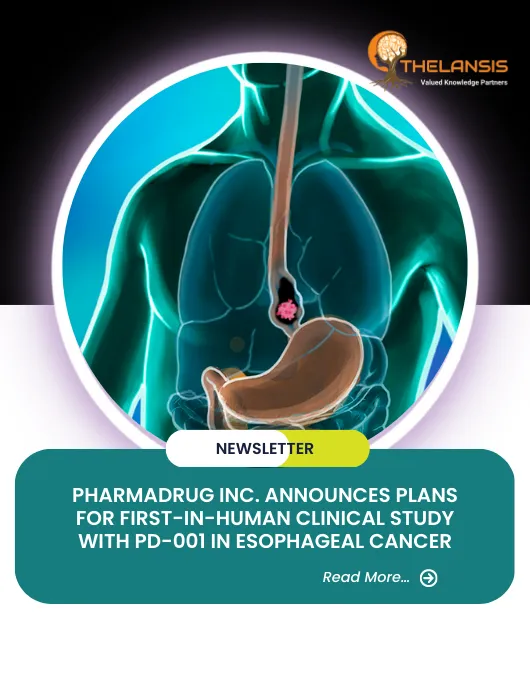
PharmaDrug Inc. Announces Plans for First-In-Human Clinical Study with PD-001 in Esophageal Cancer
PharmaDrug Inc. plans to conduct a first-in-human study of its lead candidate, PD-001, an enteric-coated cepharanthine-2HCL, in Australia in H2 2023. PD-001 has the potential as a breakthrough therapy ...
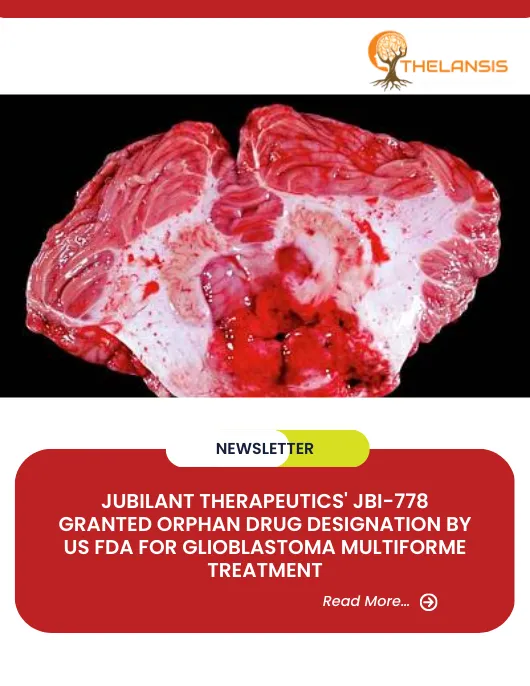
Jubilant Therapeutics’ JBI-778 Granted Orphan Drug Designation by US FDA for Glioblastoma Multiforme Treatment
Jubilant Therapeutics, a clinical-stage biopharmaceutical company, has announced that JBI-778, an oral PRMT5 inhibitor, has received orphan drug designation from the U.S. Food and Drug Administration ...

FDA Grants Orphan Drug Designation to Ezurpimtrostat (GNS561) for Hepatocellular Carcinoma (HCC) Treatment
Genoscience Pharma today announces that its lead candidate, ezurpimtrostat (GNS561), a PPT-1 (Palmitoyl Protein Thioesterase-1) inhibitor, has been granted Orphan Drug Designation by the U.S. FDA for ...

FDA Grants Orphan Drug Status to Beactica’s BEA-17 for Glioblastoma Treatment
Beactica Therapeutics, a Swedish precision oncology company, announced today that the U.S. FDA has designated BEA-17 as an orphan drug for the treatment of glioblastoma (GBM). BEA-17 is a first-of-its ...
Poxel SA Receives Orphan Drug Designation from the European Commission for PXL770 and PXL065 for Treatment of Adrenoleukodystrophy
POXEL SA, a biotech company developing treatments for serious diseases, announces the European Commission grant of orphan drug designation for PXL770 and PXL065 for Adrenoleukodystrophy treatment. The ...

FDA Approves Neuren Pharmaceutical’s Phase II Trial of NNZ-2591 for Prader-Willi Syndrome
The FDA has approved Neuren Pharmaceuticals' Phase 2 clinical trial for its NNZ-2591 drug for children with Prader-Willi syndrome, a neurological disorder with no approved treatment options. The trial ...

FDA Grants Orphan Drug Designation to HM15211 for Idiopathic pulmonary fibrosis (IPF)
Hanmi Pharmaceutical's LAPS Triple Agonist (HM15211) has been designated an orphan drug to treat Idiopathic pulmonary fibrosis (IPF). Pulmonary fibrosis affects the interstitium, the lung tissue that ...

