CyGenica Receives Orphan Drug Designation for Glioblastoma Multiforme (GBM) Treatment
CyGenica Limited, a dynamic biotech startup, has achieved a significant milestone in the fight against Glioblastoma Multiforme (GBM), an aggressive form of brain cancer. Dr. Nusrat J M Sanghamitra, Co-founder and CEO of CyGenica, announced that their groundbreaking drug conjugate for GBM treatment has received Orphan Drug Designation from the U.S. FDA. This remarkable approval marks a significant stride for CyGenica’s innovative intracellular delivery platform, GEENIE, and brings renewed hope to cancer and rare disease patients.
Publish Date: 12-09-2023 Source: CyGenica Limited
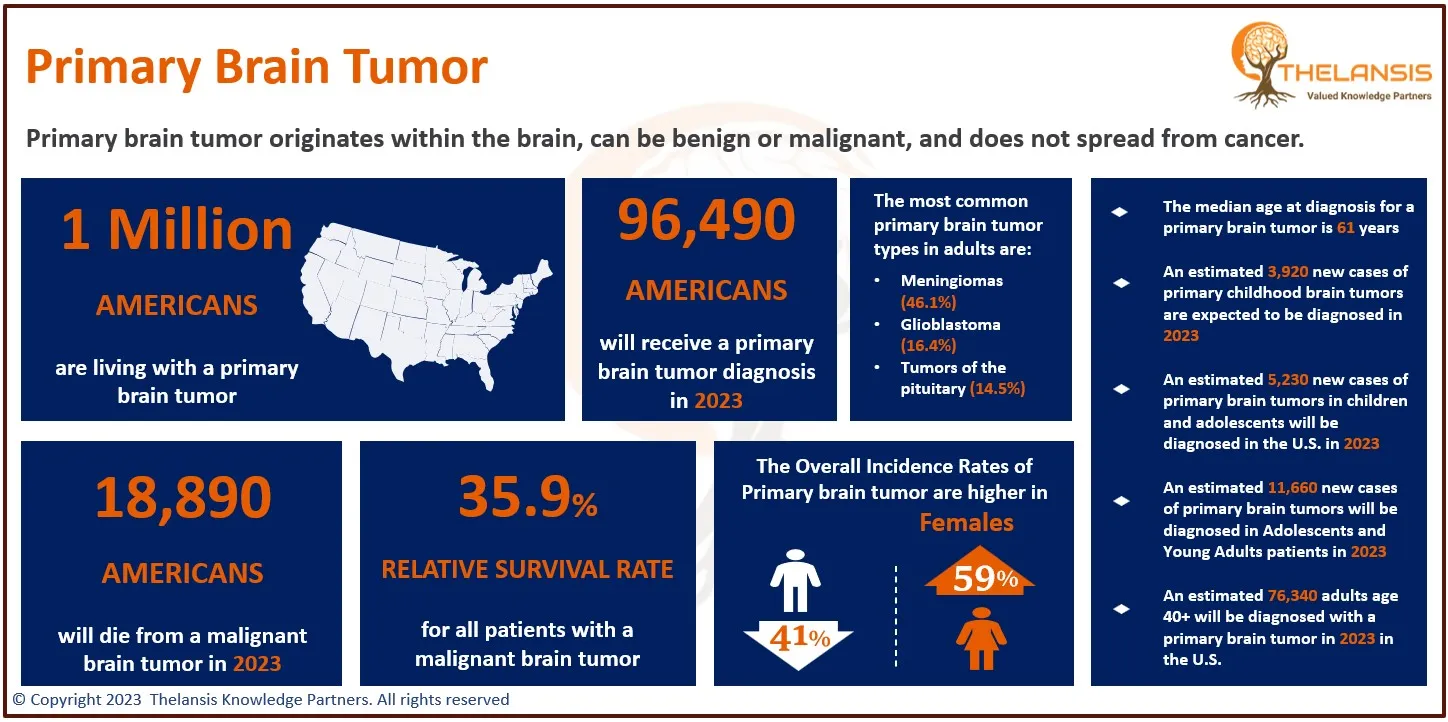
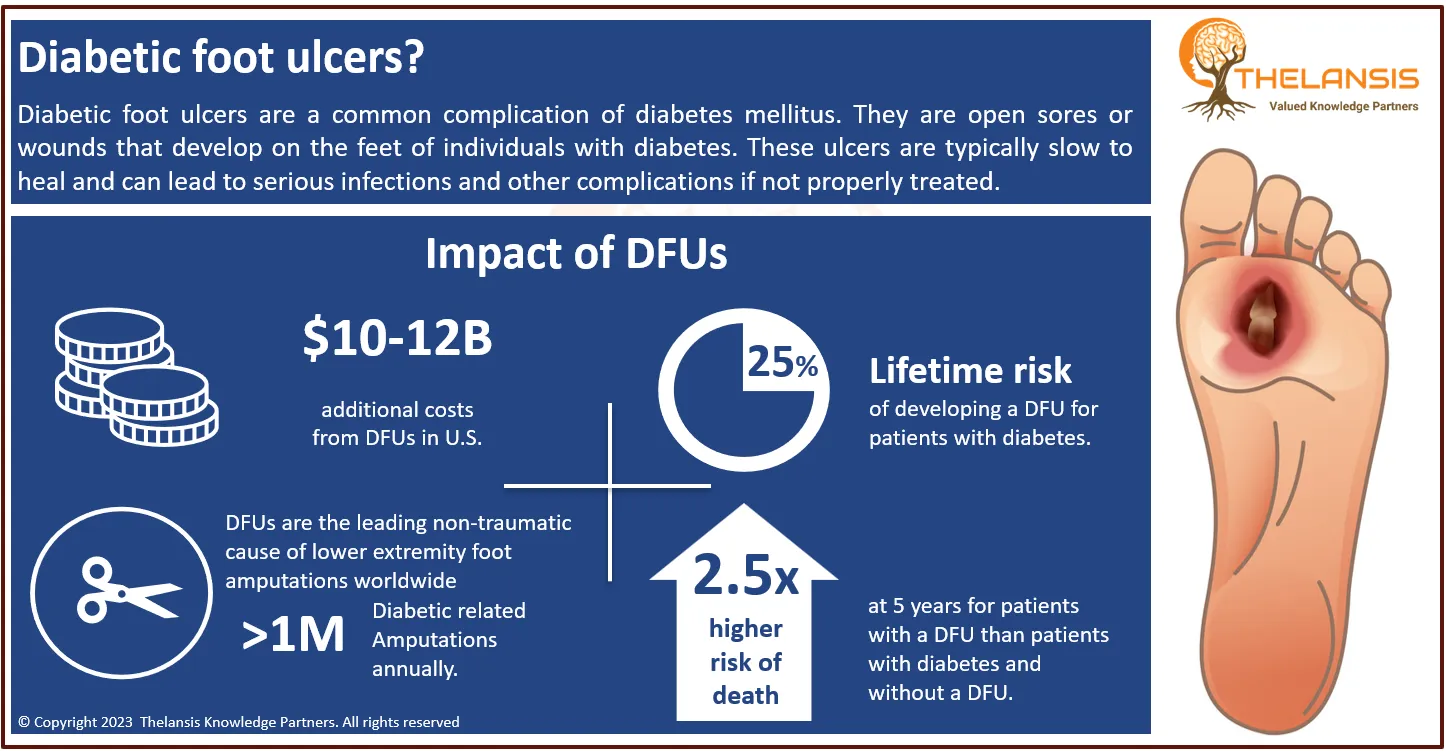
Glioblastoma multiforme (GBM) is a formidable disease, comprising 17% of adult intracranial neoplasms. No identified risk factors are associated with GBM, except for exposure to ionizing radiation. GBM is one of the most aggressive forms of cancer in humans. Despite advancements in surgical techniques and additional treatments, the average survival time remains approximately 1 year. In adults, GBM is the most prevalent type of astrocytoma and is typically associated with a grim prognosis, with a median survival of about 12 months. However, it is rare in children. Around 65% of GBM cases primarily manifest in the cerebral hemispheres, which govern higher cognitive functions such as speech, movement, thought, and sensation. GBMs can also arise in other regions of the brain that regulate sensory perception, temperature sensitivity, balance, and motor function. The most prevalent symptoms of GBM include morning headaches, lethargy, and seizures that vary depending on the type and location of the tumor. Compression of neighboring brain structures due to the tumor’s position can result in motor dysfunction, hormonal irregularities, or changes in behavior and cognitive processes. GBMs are typically diagnosed between the ages of 5 and 9, affecting both boys and girls equally. However, they occur more frequently in children with specific genetic syndromes, including neurofibromatosis 1, Li-Fraumeni syndrome, hereditary nonpolyposis colon cancer, and tuberous sclerosis. The majority of GBM cases, however, have an unknown cause. Treatment options for GBM may encompass neurosurgery, radiation therapy, and chemotherapy.
- Glioblastoma multiforme is the most aggressive malignant primary brain tumor, with an incidence of 2–3 per 100,000 people in the United States and Europe. In children, accounting for only 3% of childhood brain tumors.
However, the current Glioblastoma Multiforme (GBM) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (DSP-7888, Temozolomide, ASC40, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Glioblastoma Multiforme treatment. The key companies in the advanced development stage are Sumitomo Pharma Oncology, Inc., AbbVie, Ascletis Pharmaceuticals Co., Ltd., etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Glioblastoma Multiforme (GBM) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2022 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com