Abbisko Cayman's ABSK012 Granted FDA Orphan Drug Designation for Soft Tissue Sarcoma Treatment
Abbisko Therapeutics, a subsidiary of Abbisko Cayman, has been awarded an orphan drug designation by the US Food and Drug Administration (FDA) for ABSK012, an FGFR4 mutant inhibitor that treats soft tissue sarcoma. This designation offers numerous advantages to pharmaceutical companies, including market exclusivity for seven years after FDA approval, tax credits for qualified clinical trials, and exemption from FDA application fees.
Publish Date: 03-04-2023 Source: Abbisko Therapeutics
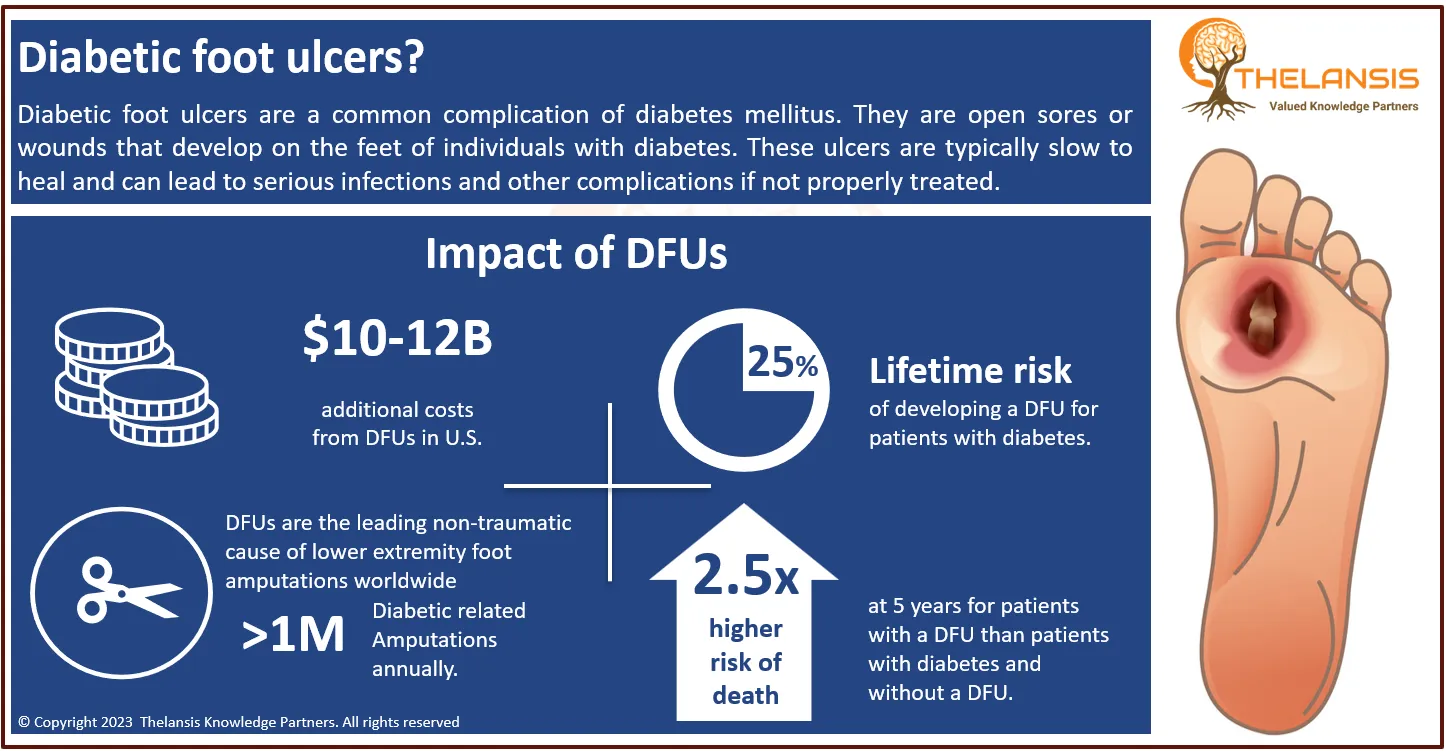
Soft tissue sarcomas (STS) are rare neoplasms, accounting for fewer than 1% of all neoplasms. More than 80 malignant or intermediate (rarely metastasizing) histotypes are currently recognized. STS is a group of malignant tumors originating in soft tissue. It includes muscle, tendons, fat, blood, and lymph vessels, nerve and joint linings. STS may occur at any age, most often in middle-aged and older adults. High-grade soft tissue sarcomas had a high mortality rate, especially in elderly patients with other comorbidities, unlike young patients with a better prognosis, which led to a better survival rate.
- Incidence of STS ranged from 3.5 to 4,7 per 100,000 population. Among all incident STS patients, 84% to 90% of patients are being diagnosed as non-metastatic, and 10 to 16% are metastatic.
However, the current Soft Tissue Sarcomas (STS) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Olaratumab, ADI PEG20, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Soft Tissue Sarcomas treatment. The key companies in the advanced development stage are Eli Lilly and Company, Polaris Group, etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Soft Tissue Sarcomas (STS) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com