According to Thelansis, the global Chemotherapy-Induced Peripheral Neuropathy (CIPN) in 8 MM countries is estimated to be USD ~2.6 BN in 2020, USD and USD 4.7 BN by the end of 2030 (10 years of markets forecasting). They attribute the high growth to the launches of Opioid analgesics, Alpha-2-delta antagonists, SNRI, Tricyclic antidepressants, analgesics, and others.
Epidemiology and patient segmentation:
According to Thelansis, the estimated total patient pool of chemotherapy-induced peripheral neuropathy (CIPN) in 8 MM countries is 3.35 MN in 2020, 3.47 MN in 2025, and 3.57 MN by 2030. The overall growth of the patient pool for CIPN in 8MM countries is mainly due to patients available to each treatment modality and the newly diagnosed patient pool.
Disease overview, Epidemiology (Incidence, prevalence, grade-wise and stage-wise), patient segmentation is reported based on the following segmentation- surgery + chemotherapy, surgery + chemotherapy + radiotherapy, chemotherapy + radiotherapy, chemotherapy only. Severity-specific incidence of CIPN is categorized based on mild, moderate, and severe conditions in the report.
Competitive Landscape:
Currently, there is no approved therapy for the treatment of CIPN. The market is mainly dominated by off-label therapies such as steroids, antidepressants, antiepileptics, opioids, and procedural therapies (Electrical nerve stimulation, Occupational therapy, Physical therapy, and Acupuncture).
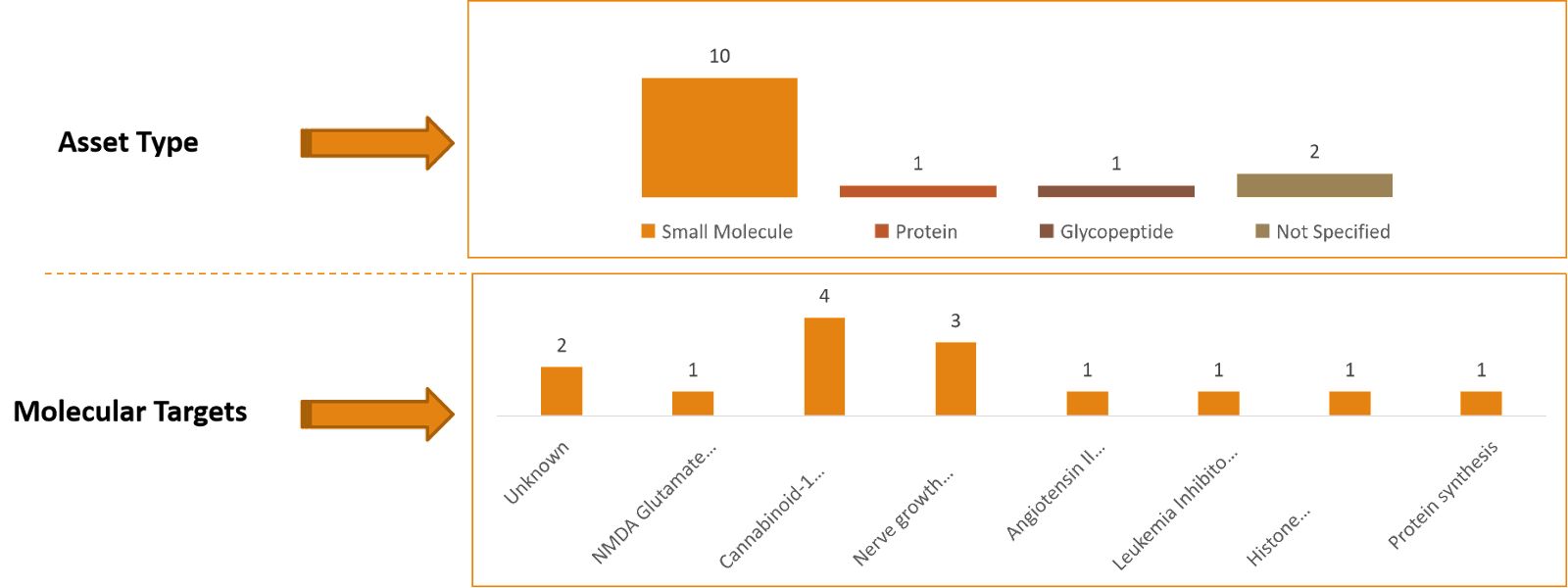
Emerging therapies: As per Thelansis, key players is into developing therapies for the treatment of CIPN are:
-
- SP-04 (PledOx) /Solasia,
- Halneuron, / Wex Pharmaceutical and
- E-52862 / Esteve Pharmaceuticals,
- ART-123 (thrombomodulin alfa) /Asahi Kasei Pharma,
- APX3330 / Apexian Pharmaceuticals.
Market Forecast: Patient Based Forecast Model (MS. Excel Based Automated Dashboard)
– Data Inputs with sourcing
– Market Event and Product Event
– Country specific Forecast Model
– Market uptake and patient share uptake
– Attribute Analysis
– Analog Analysis
– Disease burden and pricing scenario
– Summary and Insights
Landscape Developmental Overview
- Currently, there is only one asset in Phase III (PledOx) and three in Phase II. However, out of the Phase II assets, two competitors have not registered any major recent event
- Most therapies are concentrated in preclinical stages, which translates into unmet clinical needs and market opportunities.
- Overarching, there are two critical assets currently in development for CIPN with approval potential.
- Initial positive results have also generated positive sentiment around the assets. There is virtually no resonating voice for other assets considering the immediate future.

Key Opinion Leaders (KOL) Insights for
- SP-04 (PledOx),
- Halneuron, E-52862,
- ART-123 (thrombomodulin alfa),
- APX3330
KOL Opinion on following parameters,
- Comments on Drug’s Potential,
- Prognostic factors
- Clinical endpoint
- Disease burden
- Referral pattern
- Efficacy and side effects
- Unmet needs,
- Disease presentation,
- Diagnosis
- Symptomatic treatment CIPN,
- Non-pharmacological intervention,
- Reimbursement of CIPN therapy
Clinical Trial Assessment-
Detailed clinical trial data analysis and critical product positioning include trial design, primary outcomes, secondary outcomes, dosing and schedules, inclusion and exclusion criteria, recruitment status and essentially covers the reported adverse events. Majorly the trial analysis helps determine the potential of the critical assets and their probable filing and launch date.
Chemotherapy-Induced Peripheral Neuropathy (CIPN) Report TOC
| 1. CIPN – Key Findings Summary |
| 1.1. Commercial findings |
| 2. CIPN Introduction |
| 2.1 Chemotherapy-Induced Peripheral Neuropathy (CIPN) |
| 2.2 Symptoms of CIPN |
| 2.3 Grading of chemotherapy-induced peripheral neuropathy |
| 2.4 Pathophysiology of CIPN |
| 2.5 Chemotherapy-Induced Peripheral Neuropathy: Clinical Presentation |
| 2.6 Genetics of Chemotherapy-Induced Peripheral Neuropathy |
| 2.7 Diagnosis of CIPN |
| 3. Epidemiology Research |
| 3.1 Epidemiology Outlook |
| 3.2 8MM Epidemiology Outlook |
| 3.3 Country specific Epidemiology scenario |
| 3.3.1 USA Epidemiology Scenario |
| 3.3.2 EU5 Epidemiology Scenario |
| 3.3.3 Germany Epidemiology Scenario |
| 3.3.4 France Epidemiology Scenario |
| 3.3.5 Italy Epidemiology Scenario |
| 3.3.6 Spain Epidemiology Scenario |
| 3.3.7 UK Epidemiology Scenario |
| 3.3.8 Japan Epidemiology Scenario |
| 3.3.9 China Epidemiology Scenario |
| 4. Market Outlook |
| 4.1 Market Outlook |
| 4.2 8MM CIPN Market |
| 4.3 8MM CIPN Market Forecast – Till 2030 |
| 4.4 Market Forecast USA – Till 2030 |
| 4.5 Market Forecast Germany – Till 2030 |
| 4.6 Market Forecast France – Till 2030 |
| 4.7 Market Forecast Italy – Till 2030 |
| 4.8 Market Forecast Spain – Till 2030 |
| 4.9 Market Forecast UK – Till 2030 |
| 4.10 Market Forecast Japan– Till 2030 |
| 4.11 Market Forecast China – Till 2030 |
| 5. Competitive Landscape |
| 5.1 Marketed Therapies |
| 5.2 Preventive Drugs for CIPN |
| 5.3 Symptomatic Treatment of CIPN |
| 5.4 Unmet Needs |
| 5.5 Pipeline Therapies |
| 5.5.1 Phase III Therapies |
| 5.5.1.1 PledOx (Calmangafodipir) |
| 5.5.1.2 Halneuron (tetrodotoxin) |
| 5.5.1.3 APX3330 |
| 5.5.1.4 ART-123 (thrombomodulin alfa) |
| 5.5.1.5 E-52862 |
| 5.5.1.6 MN-166 (ibudilast) |
| 5.6 Pre-clinical/Research assets |
| 5.7 Inactive Therapies |
| 5.7.1 AL-309 (Endo International plc) |
| 5.8 Key Opinion Leaders (KOL) Insights |
| 5.8.1 Commentary for PledOx: |
| 5.8.2 Commentary for Halneuron: |
| 5.8.3 Commentary for E-52862: |
| 6. Regulatory Framework Scenario |
| 7. Clinical Trial Assessment – Current and Future Paradigm |
| 7.1. Distribution of Primary Endpoints across trials |
| 7.2. Distribution of Secondary Endpoints across trials |
| 7.3. Key Investigator-initiated trials |
| 7.4. Attrition Analysis |
| 8 Key Takeaways – CIPN Market |
| 8.1 Epidemiology Patterns |
| 8.2 Market Forecast |
| 9. Report Methodology |
| 10. About Thelansis |
About Thelansis:
Thelansis specializes in pharmaceutical market research and market Insight Report Company, published reports across the therapeutic area, including rare / ultra-rare and mainstream indication. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
> Delivery Office:
Bengaluru– Embassy TechVillage, Kadabeesanahalli, Bengaluru-560013 India
Gurugram– One work Gold Souk mall, Phase I, Sector 43, Haryana-122002, India
Contact no.: +91(124) 404-1731
clientsupport@thelansis.com
> Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (267) 244-6955
m.berg@thelansis.com