FDA Grants Fast Track Designation to MorphoSys AG's Promising Endometrial Cancer Treatment
MorphoSys AG today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for tulmimetostat, an innovative dual inhibitor targeting EZH2 and EZH1. This breakthrough offers hope to patients battling advanced, recurrent, or metastatic endometrial cancer with ARID1A mutations, who have already undergone at least one prior treatment. Fast Track designation from the FDA is a pivotal step, expediting the development and review process for medicines designed to address critical medical needs.
Publish Date: 12-09-2023 Source: MorphoSys AG
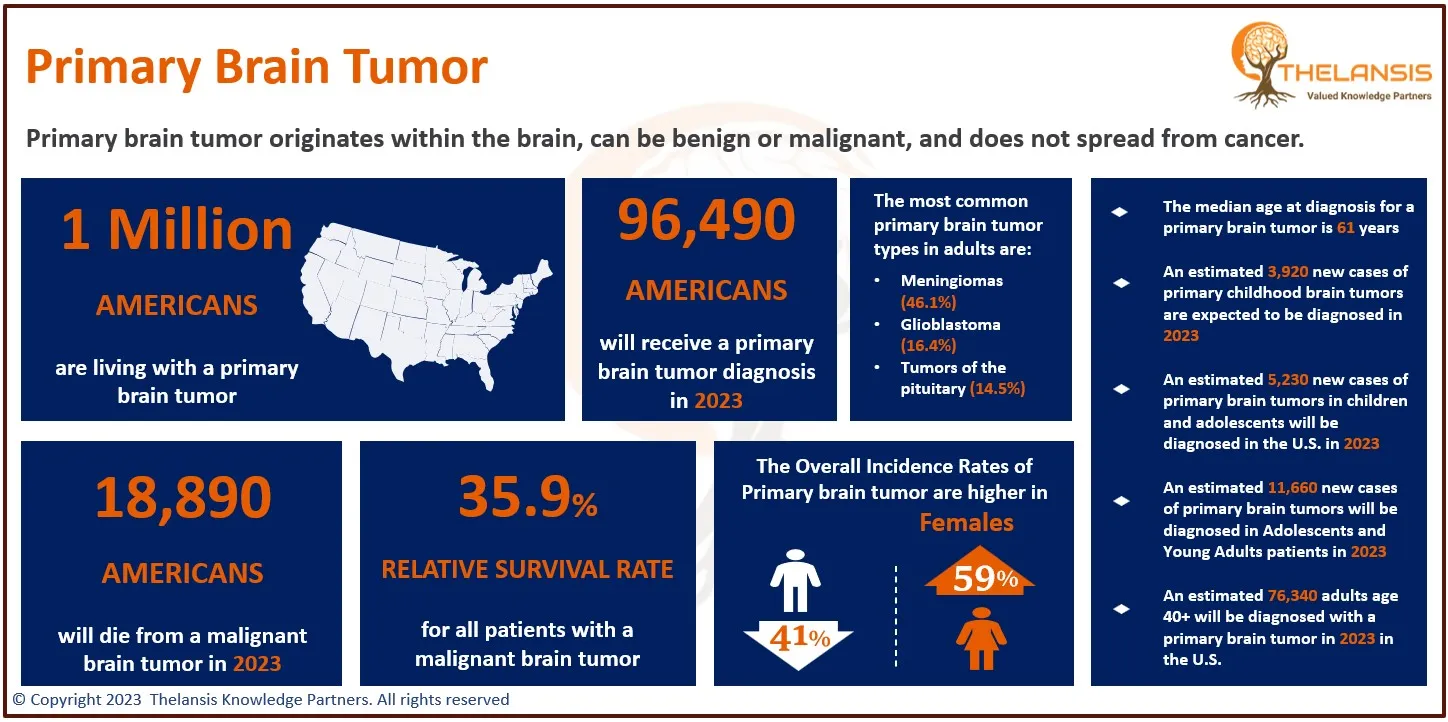
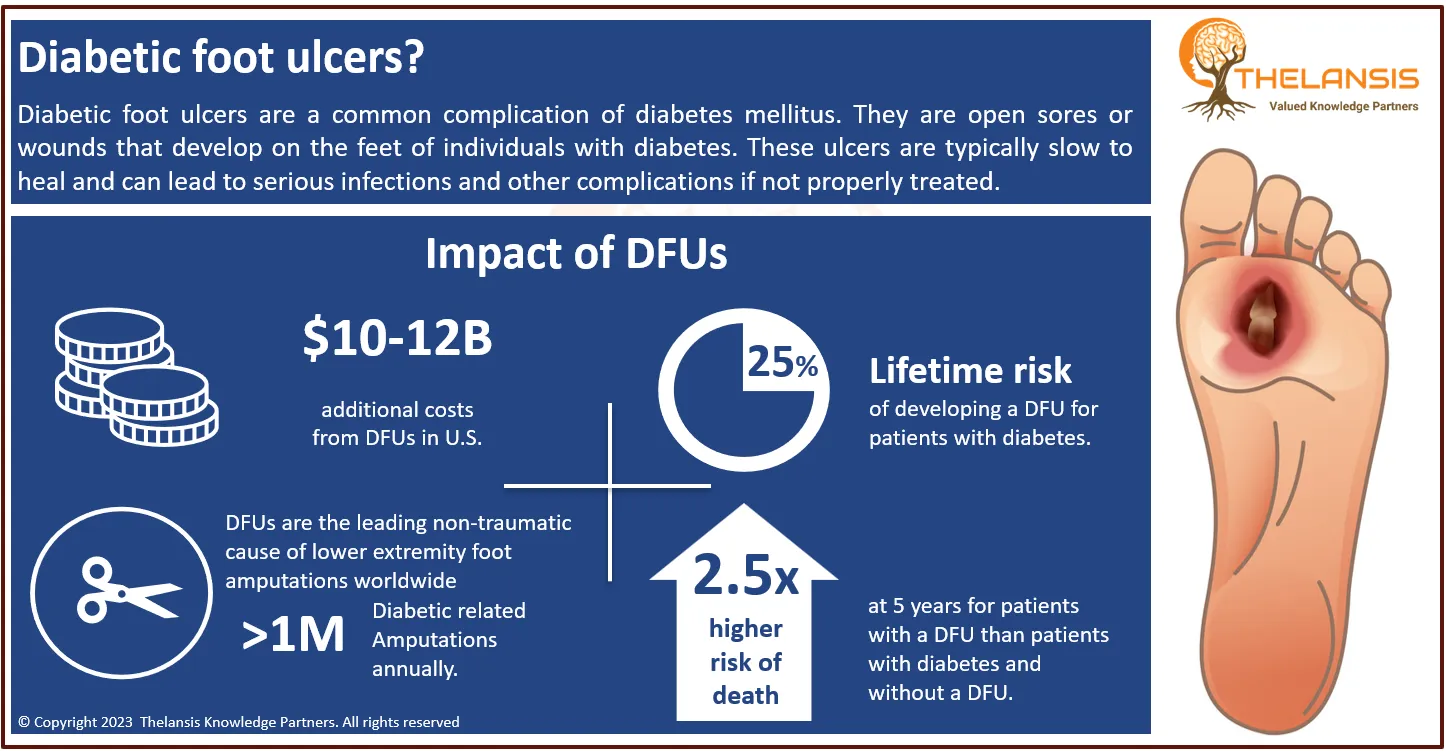
Endometrial carcinoma (EC) is the most common gynecologic malignancy that almost every gynecologist will encounter. The most common symptoms are abnormal uterine bleeding and vaginal discharge. Patients with advanced disease may have symptoms similar to those seen with advanced ovarian cancer, such as abdominal or pelvic pain, abdominal distension, early satiety, or a change in bowel or bladder function. Women with recurrent or advanced endometrial cancer constitute a heterogeneous group of patients. Most patients with endometrial cancer are diagnosed at an early stage (Stage I or II) and have a more favorable prognosis compared with patients diagnosed with advanced (Stage III or IV) disease (5-year survival rates of >74% vs. ≤15%, respectively). Prognosis is also poor for patients with distant recurrent disease (median survival of <5 years).
- The prevalence of endometrial cancer in the United States is 25.7/100,000 women per year.
However, the current Advanced or Recurrent Endometrial Cancer treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Selinexor, Lerociclib, EG-007, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Advanced or Recurrent Endometrial Cancer treatment. The key companies in the advanced development stage are SKaryopharm Therapeutics Inc, EQRx International, Inc., Evergreen Therapeutics, Inc., etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Advanced or Recurrent Endometrial Cancer to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2022 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com