Bluebird bio Receives EC Approval for SKYSONA™ for Early Cerebral Adrenoleukodystrophy (CALD)
Bluebird bio, Inc. today announced that The European Commission (EC) had approved SKYSONA™ (elivaldogene autotemcel, Lenti-D™), one-time gene therapy for the treatment of early Cerebral Adrenoleukodystrophy (CALD) in patients under the age of 18 who have an ABCD1 genetic mutation and no HLA-matched sibling hematopoietic (blood) stem cell (HSC) donor. In the European Union (EU), SKYSONA is the first one-time gene therapy approved to treat CALD.
Publish Date: 21-07-2021 Source: Bluebird bio, Inc.
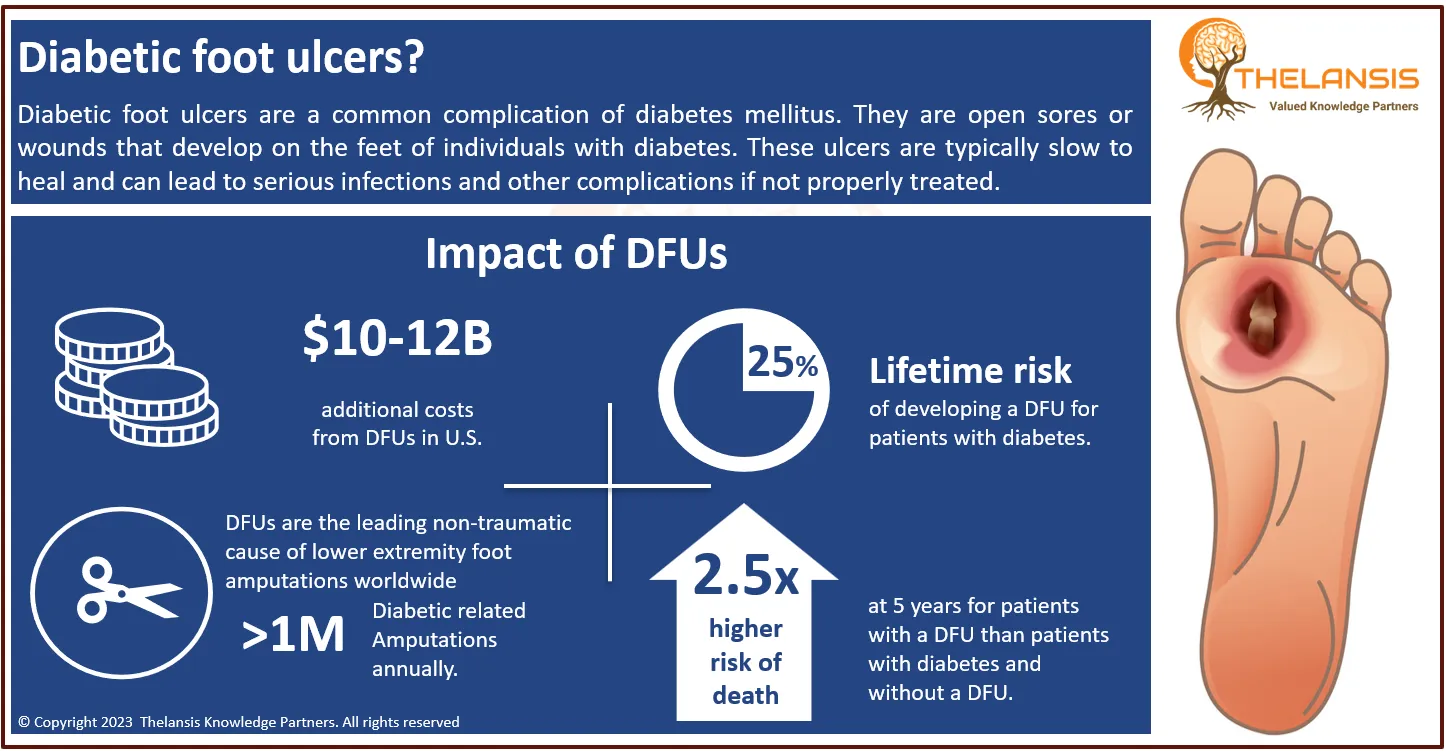
The most severe Adrenoleukodystrophy (ALD), a rare X-linked metabolic illness, is Cerebral Adrenoleukodystrophy (CALD). Between 40 and 72 percent of boys diagnosed with ALD will proceed to cerebral ALD, usually between 3 and 12. In most untreated patients, cerebral ALD is characterized by a quickly progressive neurologic decline leading to significant neurologic function loss and death. It’s a devastating condition for affected boys and their families, with severe symptom burden and rapid deterioration in neurologic function if left untreated. This is why assembling a care team to monitor your condition as soon as possible is critical.
- Based on literature research, epidemiology study, and Thelansis, the diagnosed prevalence of Adrenoleukodystrophy is approximate ~78,000 cases in 8 major markets for 2020.
However, the current Cerebral Adrenoleukodystrophy treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Lenti-D, Leriglitazone, OP-101, etc.) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Cerebral Adrenoleukodystrophy (CALD) treatment. The key companies in the advanced development stage are bluebird bio, Minoryx Therapeutics, Orpheris, etc., targeting CALD.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Cerebral Adrenoleukodystrophy (CALD) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com