Poxel SA Receives Orphan Drug Designation from the European Commission for PXL770 and PXL065 for Treatment of Adrenoleukodystrophy
POXEL SA, a biotech company developing treatments for serious diseases, announces the European Commission grant of orphan drug designation for PXL770 and PXL065 for Adrenoleukodystrophy treatment. The grant follows a positive opinion from the EMA’s COMP. The FDA has previously granted ODD and Fast Track for PXL770 and PXL065 for ALD treatment.
Publish Date: 25-01-2023 Source: POXEL SA
Adrenoleukodystrophy (ALD), also known as X-linked adrenoleukodystrophy (X-ALD), is the most common peroxisomal disorder. It is distinguished by impaired peroxisomal beta-oxidation of very long-chain fatty acids, which is reduced to about 30% of control levels. Because of mutations in the ABCD1 gene, which encodes the peroxisomal membrane protein ALDP, VLCFA accumulates in the plasma and all tissues, including the brain’s white matter, the spinal cord, and the adrenal cortex. In males with X-ALD, the clinical spectrum ranges from isolated adrenocortical insufficiency and slowly progressive myelopathy to devastating cerebral demyelination. By the age of 60, the majority of heterozygous females will have symptoms. The disease course remains unpredictable in individual patients. Based on lipid inclusions in adrenal glands, brain and Schwann cells, testis, Schaumburg, and Powers suggested the term adrenoleukodystrophy for the disorder. Adrenocortical insufficiency can be the presenting symptom of X-ALD in boys and men years or even decades before neurological symptoms appear. X-ALD is a common cause of Addison’s disease in boys and adult males, particularly when circulating adrenocortical auto is remarkably absent.
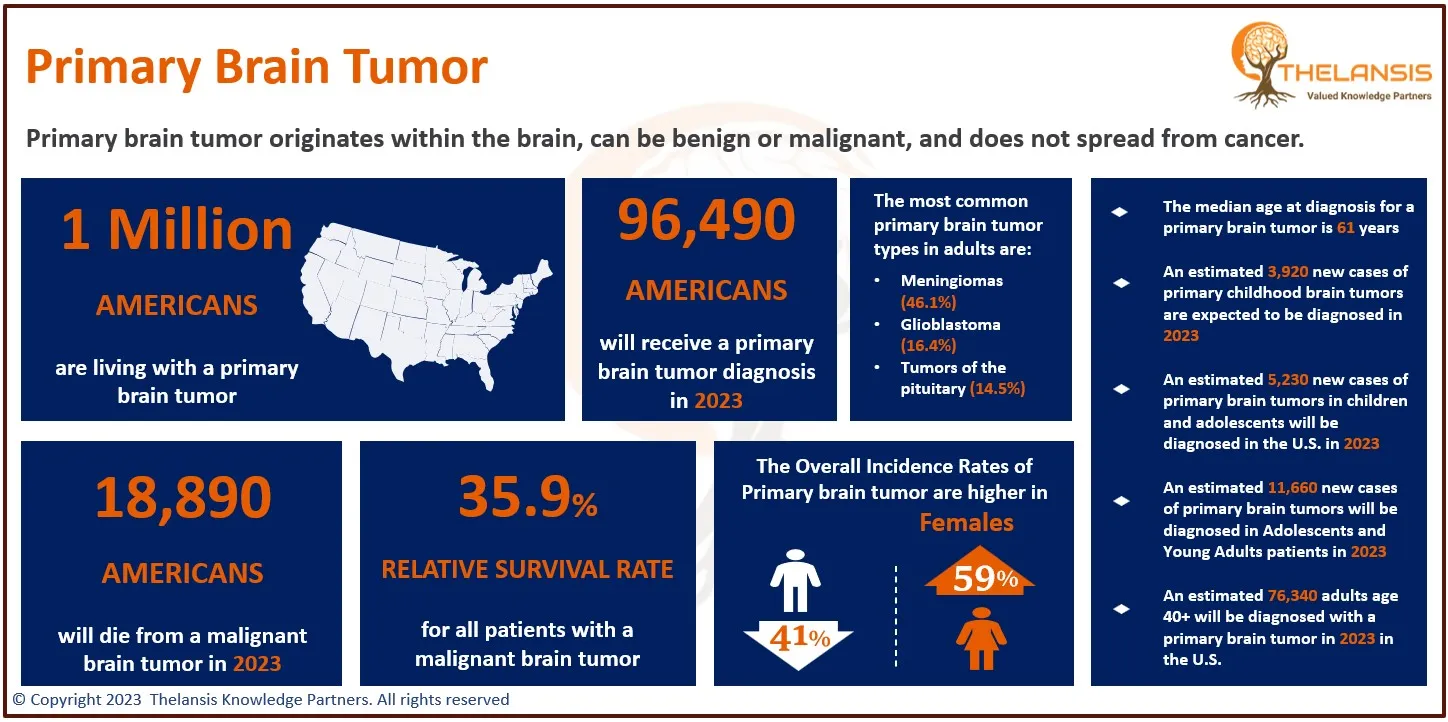
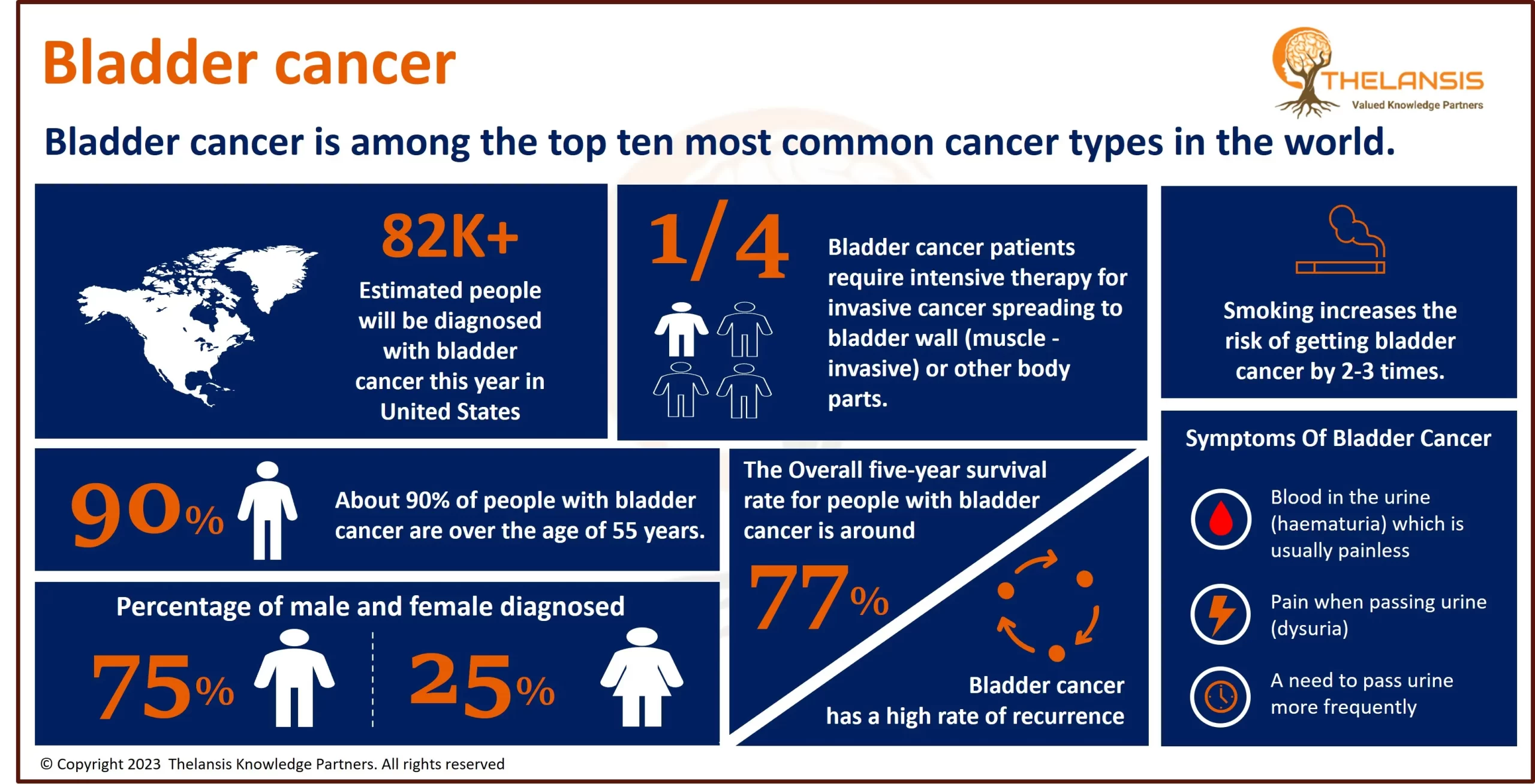
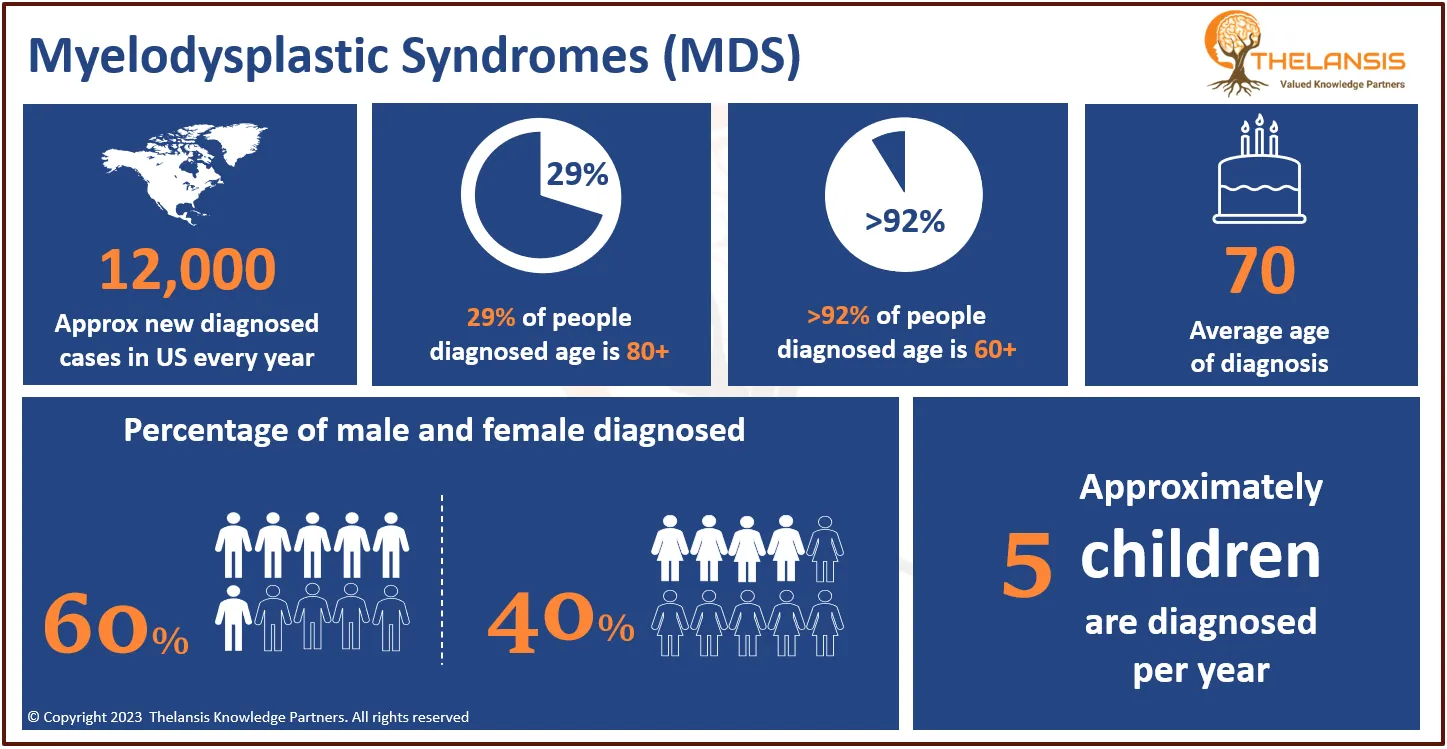
- The diagnosed incidence of X-linked adrenoleukodystrophy (X-ALD) varies between 1 to 1.5 cases per 15,000 to 20,000 live birth population in the USA.
However, the current Adrenoleukodystrophy treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (MIN-102, SBT101, etc.) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
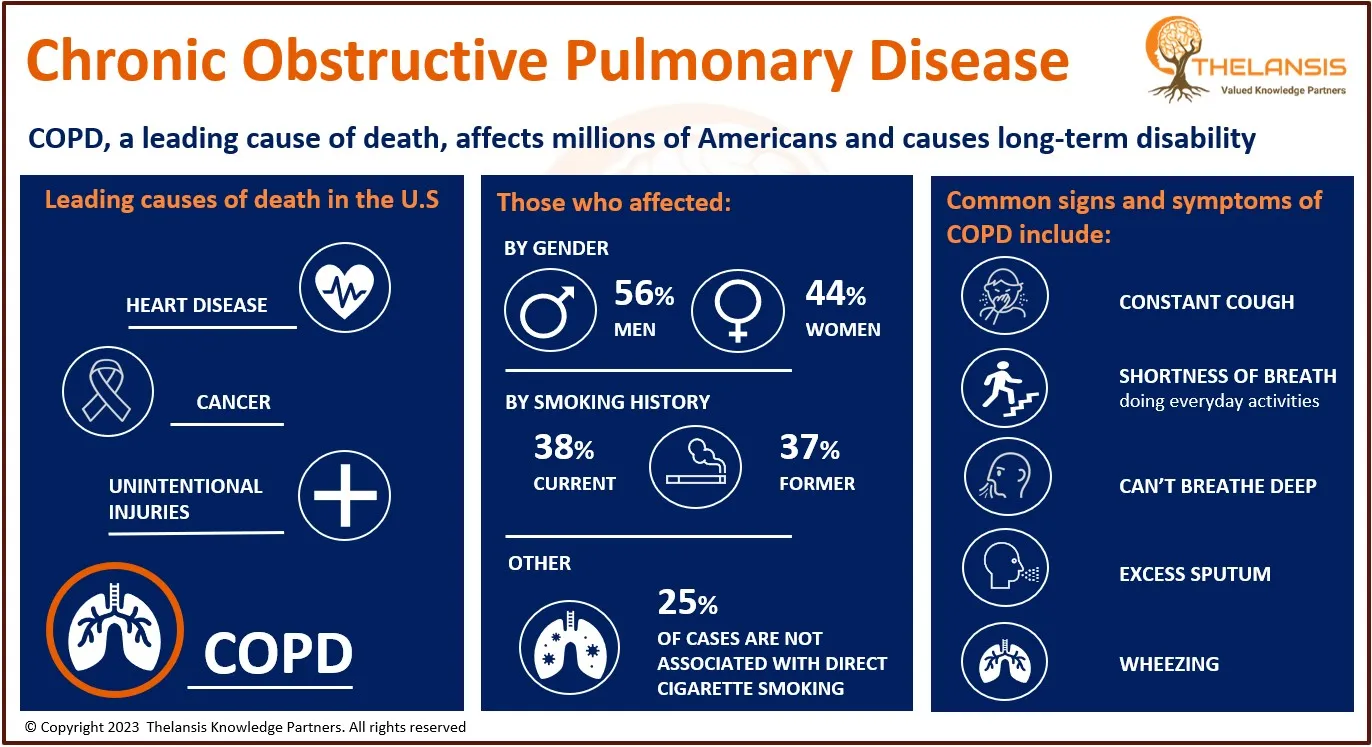
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Adrenoleukodystrophy treatment. The key companies in the advanced development stage are Minoryx Therapeutics, S.L., SwanBio Therapeutics, Inc., etc., targeting ALD.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Adrenoleukodystrophy to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com