Abbisko Cayman’s ABSK012 Granted FDA Orphan Drug Designation for Soft Tissue Sarcoma Treatment
Abbisko Therapeutics, a subsidiary of Abbisko Cayman, has been awarded an orphan drug designation by the US Food and Drug Administration (FDA) for ABSK012, an FGFR4 mutant inhibitor that treats soft t ...
Caribou Biosciences’ CB-011 Granted Fast Track Designation by FDA for Relapsed or Refractory Multiple Myeloma
Caribou Biosciences Ltd., a top-notch clinical-stage biopharmaceutical company specializing in CRISPR genome-editing, has recently received Fast Track designation from the U.S. Food and Drug Administr ...
Renovion’s ARINA-1 Receives Fast Track Designation from FDA for Prevention of BOS Progression in Bilateral Lung Transplant Patients
Renovion, Inc. has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for ARINA-1, a medicine developed for the prevention of bronchiolitis obliterans syndrome (BOS) prog ...
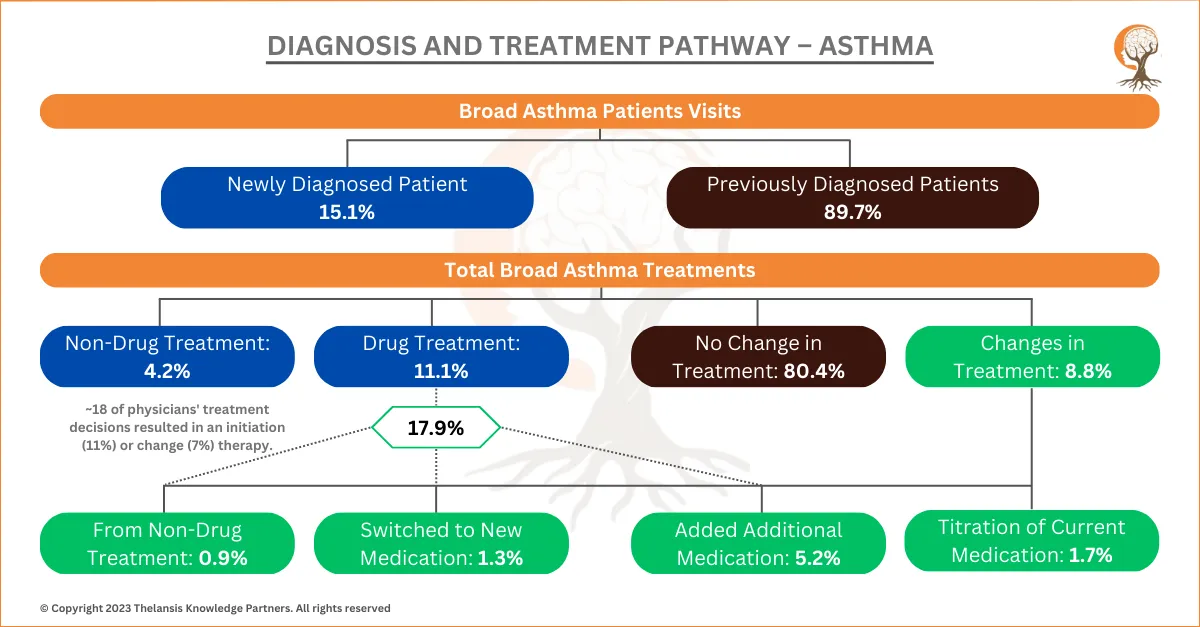
Diagnosis and Treatment Pathway – Asthma
[vc_row][vc_column][vc_custom_heading text="Diagnosis and Treatment Pathway - Asthma" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_c ...
FDA Grants Fast Track Designation to EpicentRx’s RRx-001 for Severe Oral Mucositis Prevention in Head & Neck Cancer Patients
EpicentRx, Inc. has announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to RRx-001, a novel compound with direct NLRP3 inhibitory and Nrf2 upregulatory effect ...

AnGes’ Lonafarnib Granted Orphan Drug Designation by Japanese Ministry of Health for HGPS Treatment
Anges Inc. announced that Lonafarnib (marketed in the US as Zokinvy), a medication designed to treat Hutchinson-Gilford Progeria Syndrome (HGPS) and Processing-Deficient Progeroid Laminopathy (PL), ha ...
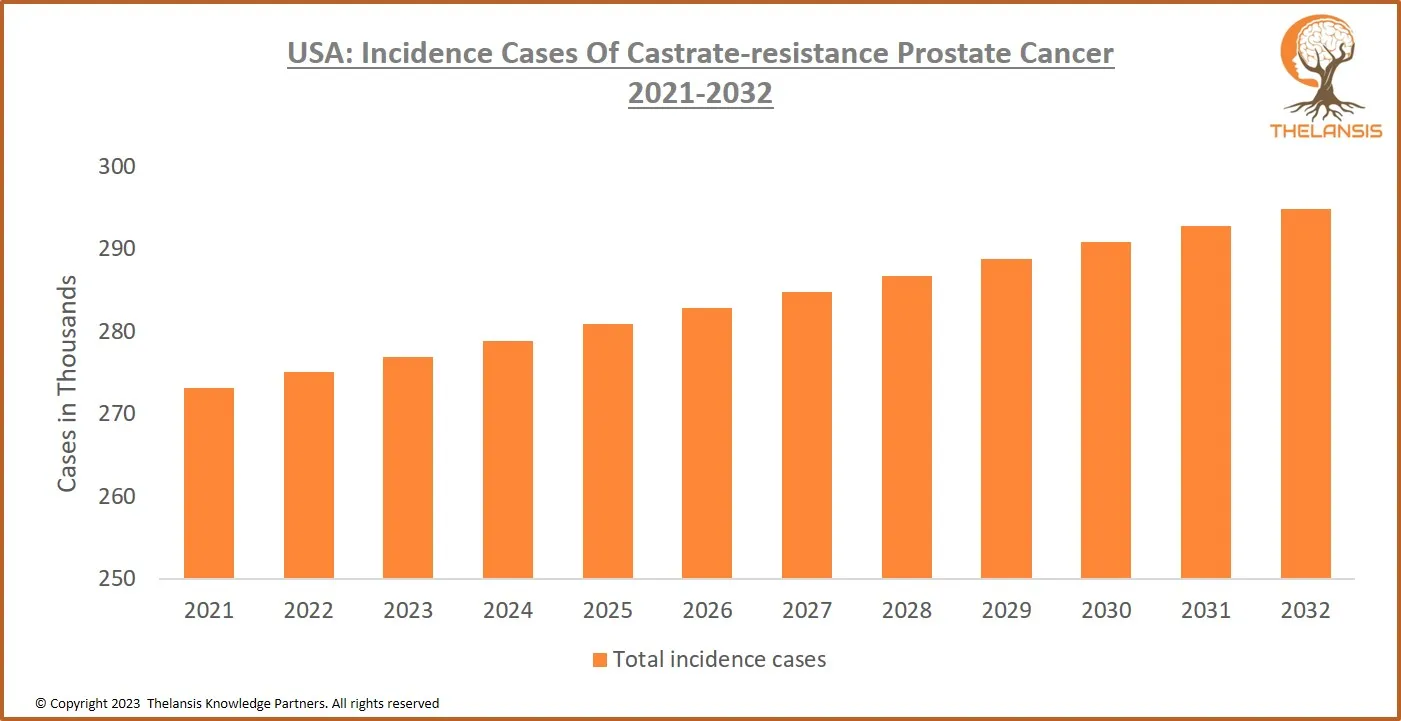
USA: Incidence Cases of Castrate-Resistant Prostate Cancer 2021-2032
[vc_row][vc_column][vc_custom_heading text="USA: Incidence Cases of Castrate-Resistant Prostate Cancer 2021-2032" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][ ...

Pharming Group’s Joenja® receives FDA approval for the treatment of rare APDS condition in Adults and Pediatric patients
Pharming Group N.V. has received FDA approval for Joenja® (leniolisib) to treat activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in patients aged 12 and above. Joenja® is a selective ...

Stoke Therapeutics Receives FDA Approval to Administer Higher Single Dose of STK-001 in MONARCH Trial for Dravet Syndrome
Stoke Therapeutics' experimental therapy for Dravet syndrome is being tested in the U.S.-based Phase 1/2a MONARCH trial, where a higher single dose of STK-001 will be administered. The U.S. Food and D ...
FDA Grants Orphan Drug Status to Zydus Lifesciences’ ZYIL1 for Treating Cryopyrin-Associated Periodic Syndrome
Zydus Lifesciences has announced that its drug, ZYIL1, has received orphan drug designation (ODD) from the United States Food and Drug Administration (US FDA) for the treatment of Cryopyrin Associated ...

