FDA Grants Fast Track Designation to EpicentRx's RRx-001 for Severe Oral Mucositis Prevention in Head & Neck Cancer Patients
EpicentRx, Inc. has announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to RRx-001, a novel compound with direct NLRP3 inhibitory and Nrf2 upregulatory effects. The drug possesses anti-inflammatory and antioxidant properties and is intended for the prevention and attenuation of severe oral mucositis in head and neck cancer patients undergoing chemotherapy and radiation.
Publish Date: 29-03-2023 Source: EpicentRx, Inc.
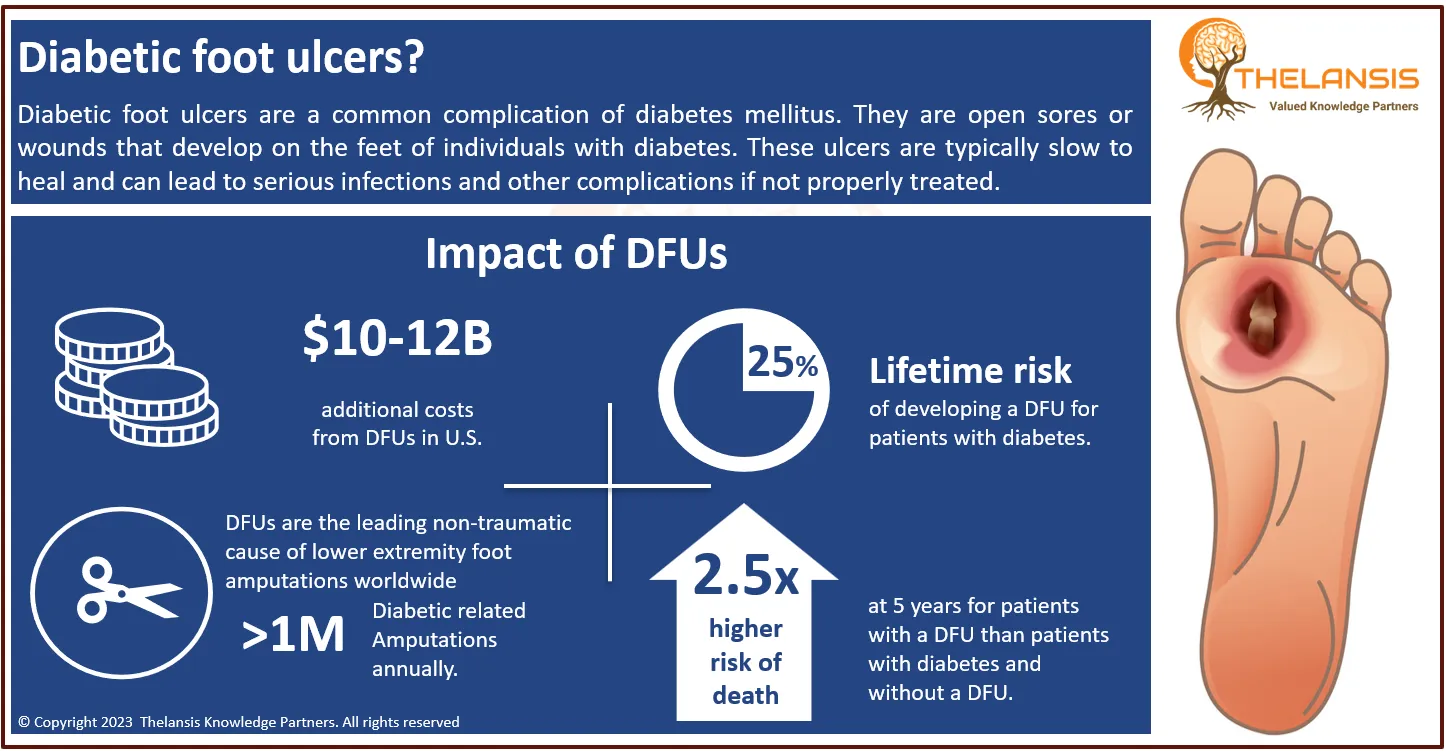
Oral Mucositis (OM), also called stomatitis, is the most common, debilitating complication of cancer chemotherapy and radiotherapy, characterized by inflammation and ulceration in the oral cavity caused by the chemotherapeutic drug substances and radiotherapy used in cancer treatment. Typical manifestations of Oral Mucositis are varying degrees of erythema, atrophy, ulceration, and mucosa swelling. It has a significant negative impact on patient’s quality of life (QOL). Potential complications of OM include pain, increased risk of local and systemic infections, bleeding, insufficient food intake, delays in the administration of radiotherapy and or chemotherapy, a dose reduction of the chemotherapy drugs, increased length of hospital stays, associated economic burdens and in some cases life-threatening infections (septicemia in neutropenic instances). Mucositis is one of the most generally reported toxicities of mTOR inhibitors, and in some studies, it was the dose-limiting toxicity. Oral toxicities with other targeted agents, such as kinase inhibitors and monoclonal antibodies, are less common. Yet studies have shown a significant incidence of all-grade mucositis with bevacizumab, erlotinib, sorafenib, and sunitinib.
- The incidence of oral toxicity associated with targeted agents has been shown to increase as the dose increases. The incidence of Oral Mucositis (OM) ranges from 590 to 650 cases per 100,000 population in the USA.
However, the current Oral Mucositis treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (GC4419, Clonidine HCl MBT, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Oral Mucositis treatment. The key companies in the advanced development stage are Galera Therapeutics, Inc., Monopar Therapeutics, etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Oral Mucositis to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com