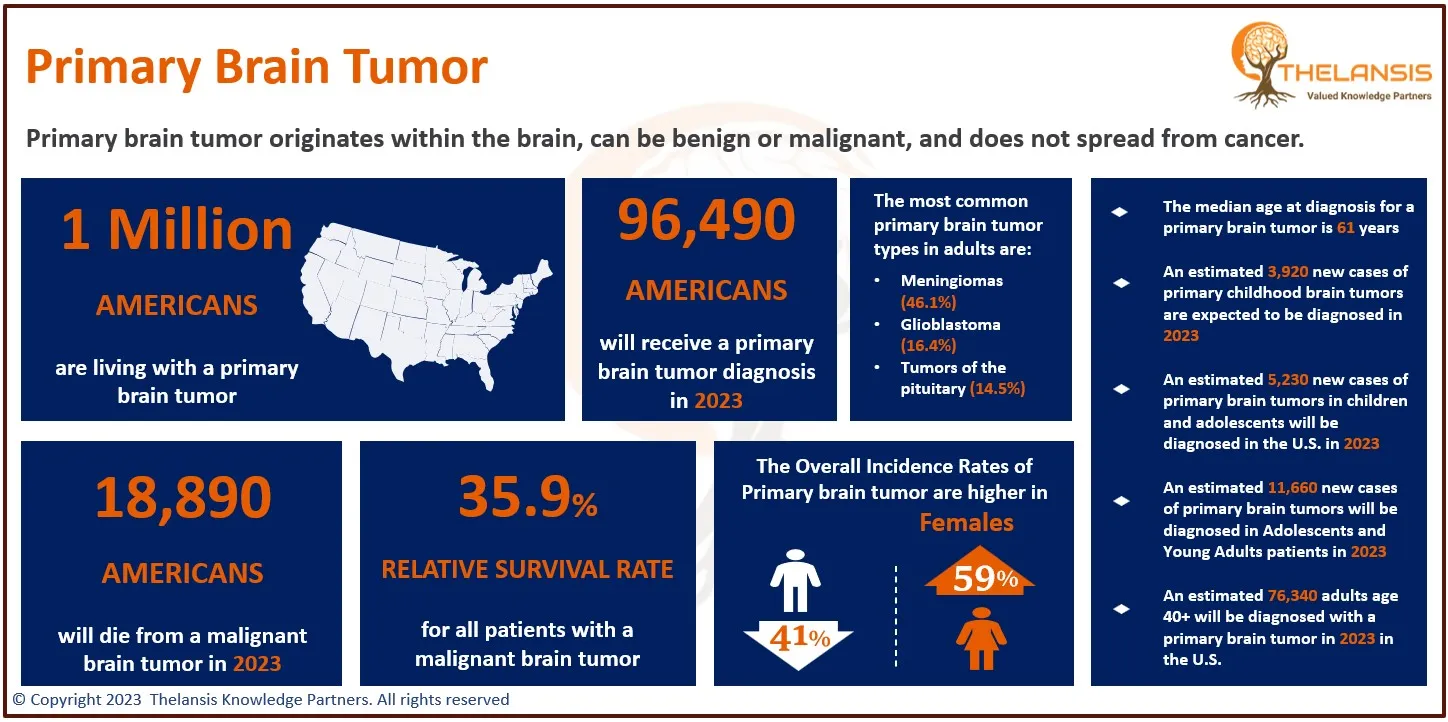
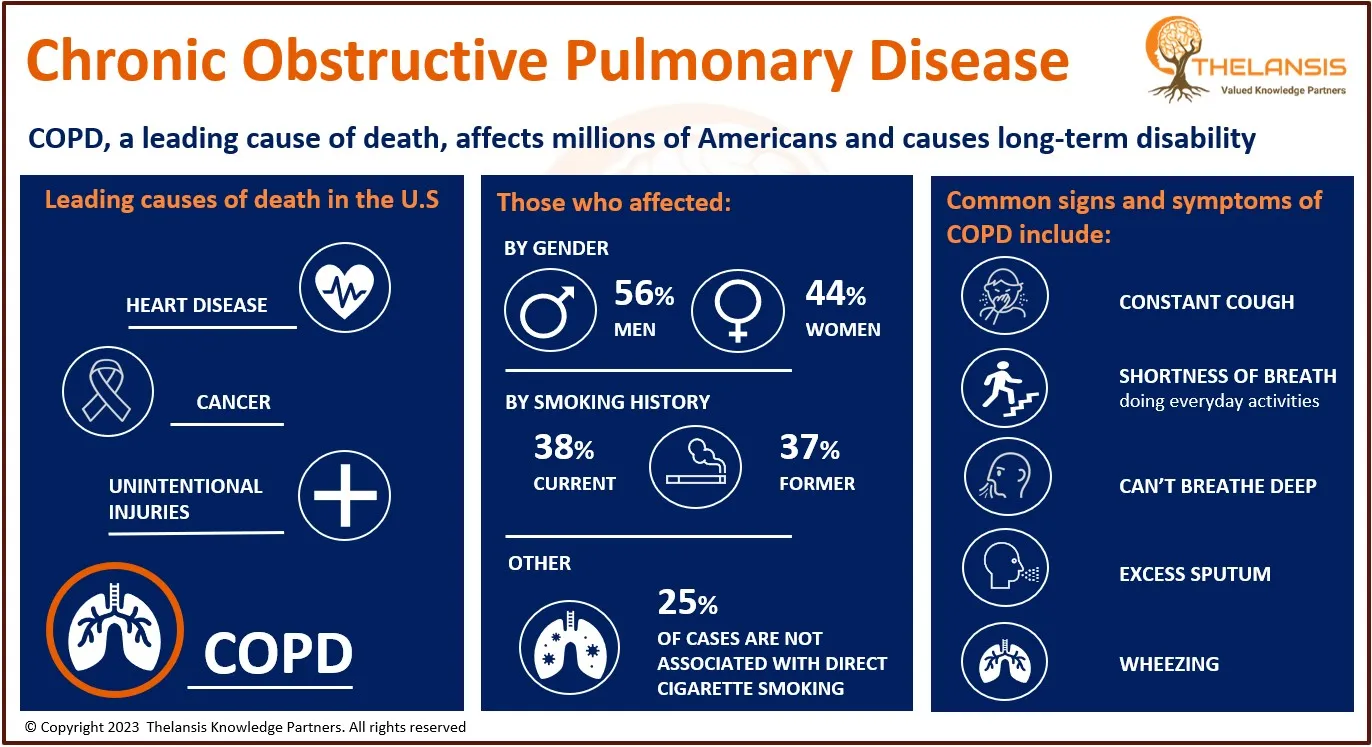
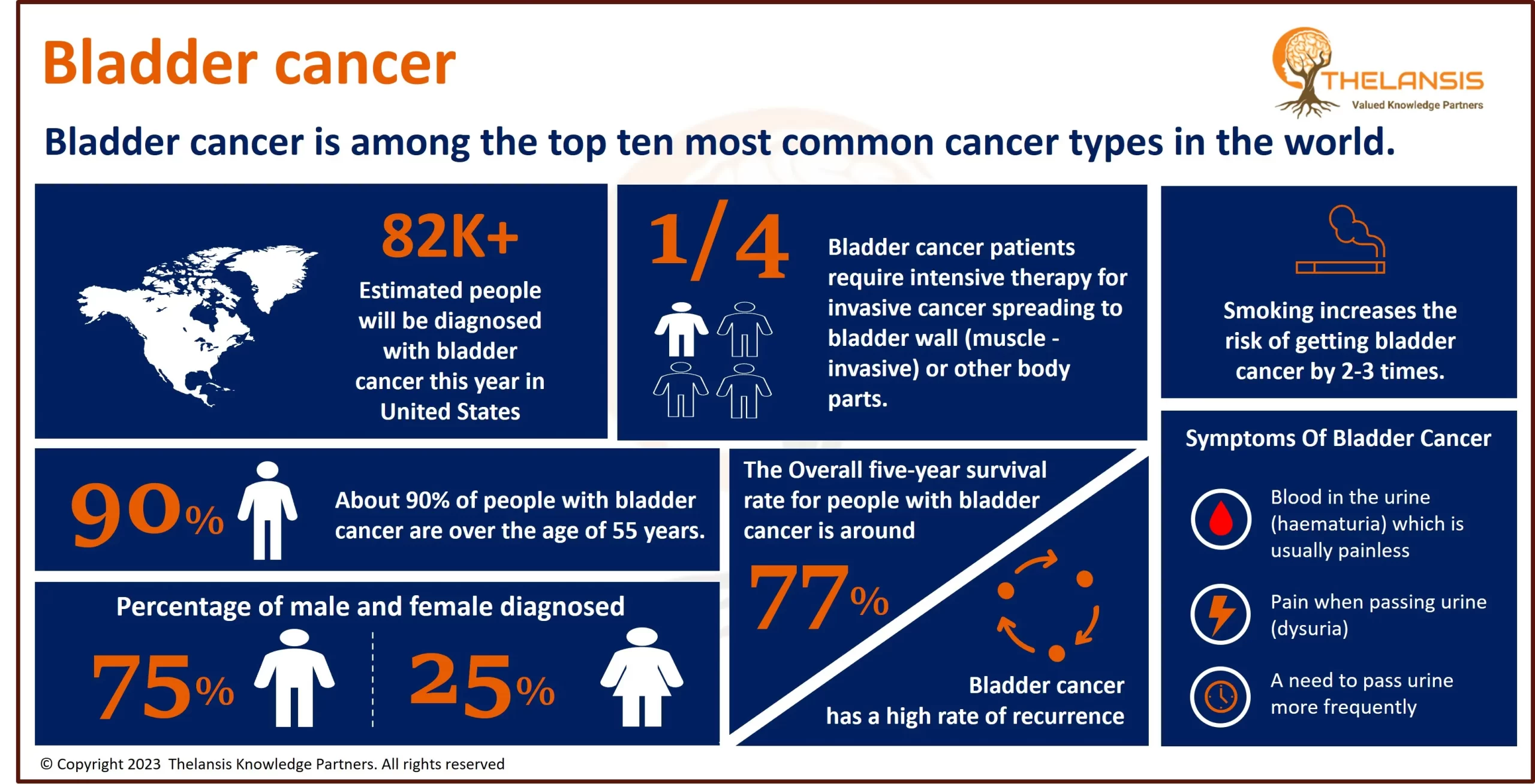
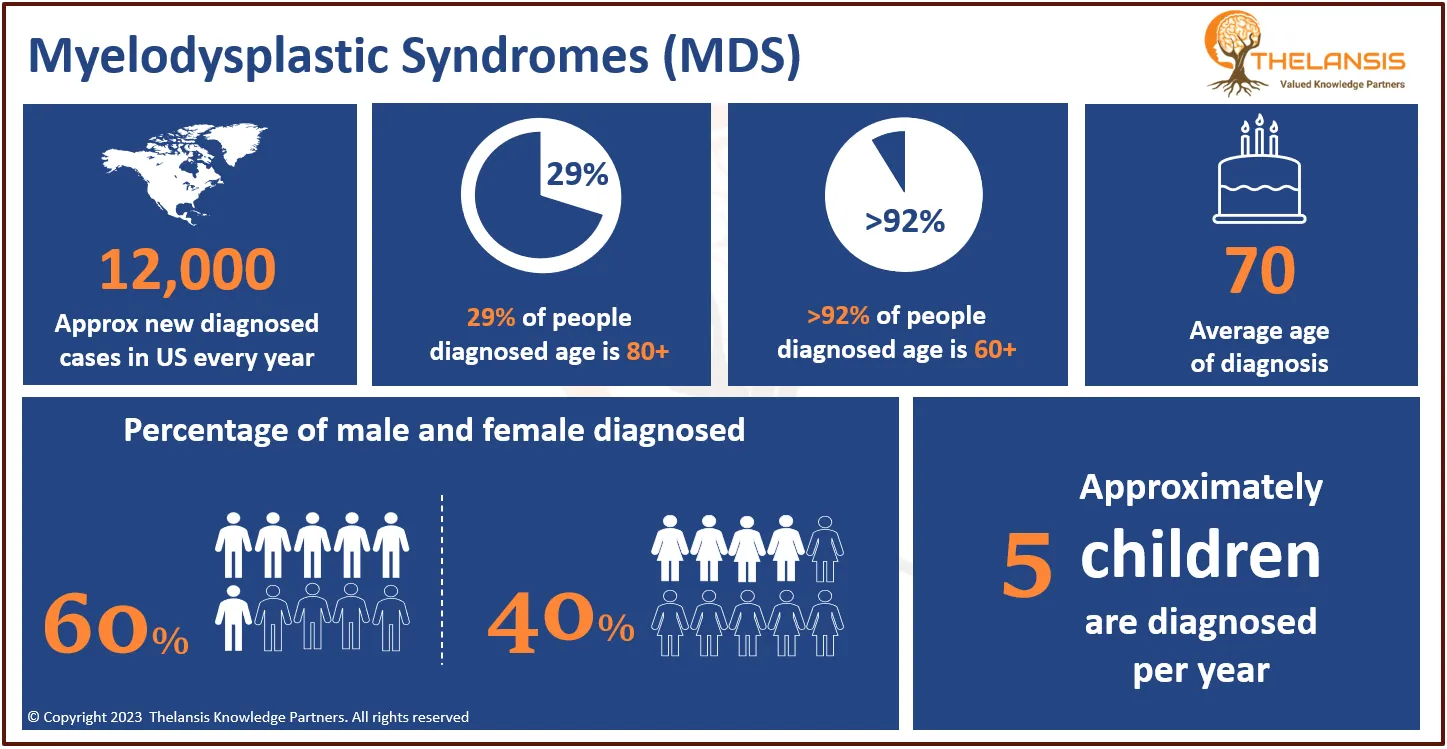
AnGes' Lonafarnib Granted Orphan Drug Designation by Japanese Ministry of Health for HGPS Treatment
Anges Inc. announced that Lonafarnib (marketed in the US as Zokinvy), a medication designed to treat Hutchinson-Gilford Progeria Syndrome (HGPS) and Processing-Deficient Progeroid Laminopathy (PL), has been designated as an orphan drug by the Japanese Ministry of Health, Labour and Welfare. They are currently preparing to submit an early approval application for the drug and are proud to receive this recognition for our efforts.
Publish Date: 28-03-2023 Source: Anges Inc.
Hutchinson-Gilford progeria syndrome (HGPS), also called progeria, is a rare, fatal genetic childhood condition with striking features resembling premature aging. Children with progeria usually have a regular appearance in early infancy. At approximately nine to 24 months, affected children experience profound growth delays, resulting in short stature and low weight. Characteristic facial features include a head that is disproportionately large for the face, narrow nasal ridge, narrow nasal tip, thin vermilion of the upper and lower lips, small mouth, and retro- and micrognathia. Standard features include loss of subcutaneous fat, delayed eruption and loss of primary teeth, abnormal skin with small outpouchings over the abdomen and upper thighs, alopecia, nail dystrophy, coxa valga, and progressive joint contractures. Later findings include low-frequency conductive hearing loss, dental crowding, and partial lack of secondary tooth eruption. Motor and mental development are typical. Death occurs due to complications of severe atherosclerosis, either cardiac disease (myocardial infarction or heart failure) or cerebrovascular disease (stroke), generally between ages six and 20. The average life span is approximately 14.5 years. The skeletal defects observed in children with HGPS are unique and characteristic. These may include delayed closure of the “soft spot” at the front of the skull, an abnormally thin portion, and/or the absence of certain air-filled cavities within the head that open into the nose.
- The prevalence of Progeria or Hutchinson-Gilford progeria syndrome (HGPS) is approximately 1 to 2 cases per 20 million, with around 550 to 650 children living with progeria worldwide.
However, the current Hutchinson-Gilford Progeria Syndrome (HGPS) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Zoledronic , Progerinin, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Hutchinson-Gilford Progeria Syndrome treatment. The key companies in the advanced development stage are Merck Sharp & Dohme LLC, PRG Science & Technology Co., Ltd., etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Hutchinson-Gilford Progeria Syndrome (HGPS) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com