Prestige Biopharma’s PBP1510 Granted Fast Track Designation by FDA for Advanced Pancreatic Cancer Treatment
Prestige Biopharma, a leading biopharmaceutical company, has secured Fast Track designation from the U.S. Food and Drug Administration (FDA) for its innovative drug, PBP1510 (International Non-proprie ...
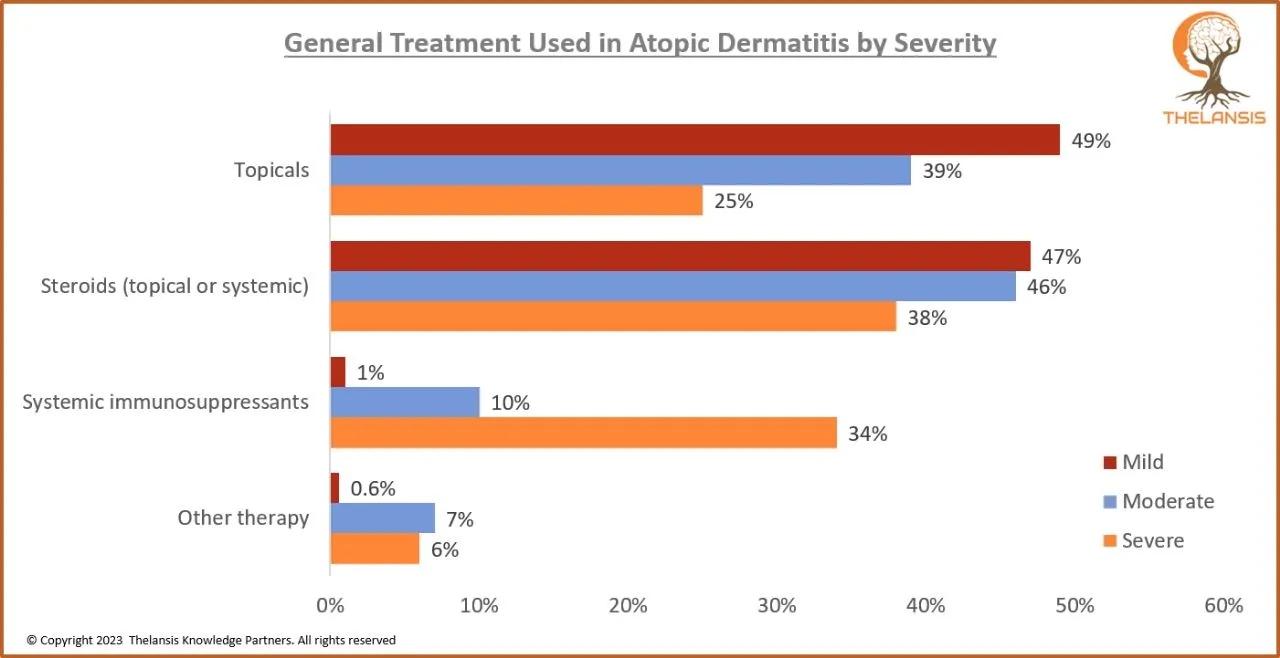
General Treatment used in Atopic Dermatitis by Severity
[vc_row][vc_column][vc_custom_heading text="General Treatment used in Atopic Dermatitis by Severity" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_co ...

Aldeyra Therapeutics Completes Enrollment for Phase 2 trial of ADX-2191 for Treatment of Retinitis Pigmentosa
Aldeyra Therapeutics, Inc. has announced the completion of the enrollment process for the Phase 2 Clinical Trial of ADX-2191 (methotrexate injection, USP), a potential treatment for retinitis pigmento ...
Vorasidenib Receives FDA Fast-Track Designation in Phase 3 INDIGO Trial for IDH-Mutant Low-Grade Glioma Treatment
Servier announced that its Vorasidenib has been granted FDA fast-track designation for its ability to prolong progression-free survival (PFS) and time to next intervention (TTNI) in patients with resi ...
Everest Medicines’ Partner Calliditas Therapeutics Reports Positive Topline Results for Nefecon in Phase 3 Clinical Trial for Primary IgA Nephropathy
Everest Medicines' partner, Calliditas Therapeutics AB, has reported positive topline results from the global Phase 3 clinical trial of NefIgArd. The trial investigated the effects of Nefecon versus p ...
Ellipses Pharma’s EP0042 Granted Orphan Drug Designation by FDA for AML Treatment
Ellipses Pharma obtains Orphan Drug Designation (ODD) from the FDA for EP0042, a dual FLT-3 and Aurora kinase inhibitor, as a treatment for acute myeloid leukaemia (AML). This designation is granted t ...
Pliant Therapeutics Initiates Phase 2a Trial Enrollment of Bexotegrast for Primary Sclerosing Cholangitis Treatment
Pliant Therapeutics, a biopharmaceutical company focused on treating fibrosis, has begun Phase 2a enrollment for its bexotegrast to treat primary sclerosing cholangitis (PSC). The trial follows a posi ...

Acadia Pharmaceuticals’ DAYBUE™ (trofinetide) Receives FDA Approval for Rett Syndrome Treatment
Neuren Pharmaceuticals has announced that its North American partner, Acadia Pharmaceuticals, has been granted FDA approval for the use of DAYBUE™ (trofinetide) in treating Rett syndrome among pediatr ...
AB2 Bio Completes Enrolment for Phase 3 Study of Tadekinig Alfa, a Novel Therapy for Primary Monogenic IL-18 Driven HLH
AB2 Bio Ltd. has completed enrolling participants in its Phase 3 study of Tadekinig alfa, a treatment for primary monogenic IL-18 driven HLH, an ultra-rare and life-threatening condition affecting chi ...
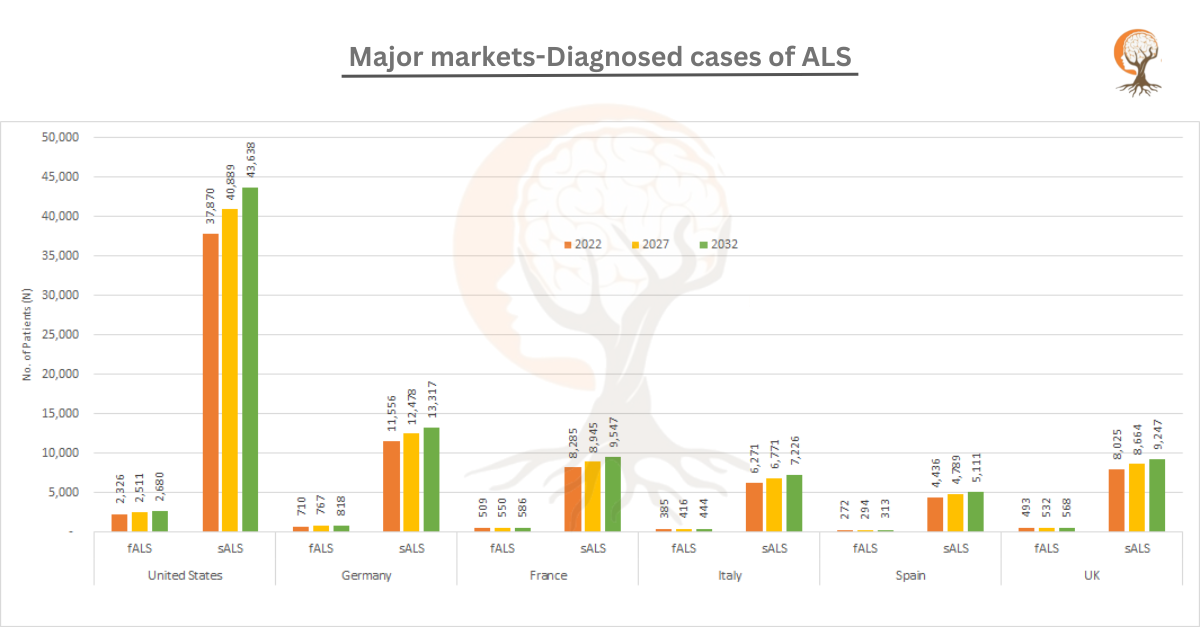
Major markets-Diagnosed cases of ALS
[vc_row][vc_column][vc_custom_heading text="Major markets-Diagnosed cases of ALS" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custo ...

