Rezolute, Inc. Receives FDA Orphan Drug Designation for Ersodetug for Hypoglycemia Due to Tumor Hyperinsulinism
Rezolute, Inc., a leading late-stage biopharmaceutical company focused on developing transformative therapies for rare diseases, announced that the U.S. Food and Drug Administration (FDA) has granted ...

Aquestive Therapeutics Receives Positive FDA Feedback for Anaphylm™ (Epinephrine) Sublingual Film
Aquestive Therapeutics Receives Positive FDA Feedback for Anaphylm™ (Epinephrine) Sublingual Film Aquestive Therapeutics, Inc., a trailblazing pharmaceutical company dedicated to improving patients ...
FDA Fast Tracks FELIQS’ FLQ-101 for Preventing Retinopathy of Prematurity (ROP)
The U.S. Food and Drug Administration (FDA) has granted fast-track designation to FELIQS' lead product, FLQ-101, which aims to prevent retinopathy of prematurity (ROP). FLQ-101 can be administered ora ...
FDA Accepts UroGen’s NDA for UGN-102, Potential First Treatment for LG-IR-NMIBC
UroGen Pharma Ltd., a biotech company developing and commercializing innovative solutions for urothelial and specialty cancers, has announced the FDA acceptance of the New Drug Application (NDA) for U ...
European Commission Grants Orphan Designation to Theriva Biologics’ VCN-01 for Retinoblastoma
Theriva Biologics, a clinical-stage biotech company developing cancer therapeutics, announced that the European Commission has adopted the EMA's recommendation to grant orphan medicinal product design ...
Capricor Therapeutics Begins BLA Submission for Deramiocel to Treat DMD Cardiomyopathy
Capricor Therapeutics, a biotechnology company dedicated to developing transformative cell and exosome-based therapeutics for rare diseases, has announced the initiation of its rolling submission proc ...

FDA Grants Breakthrough Therapy Designation to Mirum’s Volixibat for Cholestatic Pruritus in PBC
Mirum Pharmaceuticals, Inc. has announced that the U.S. Food and Drug Administration (FDA) has awarded Breakthrough therapy designation to their drug, volixibat, as a potential treatment for cholestat ...

FDA Grants Fast Track Designation to MorphoSys AG’s Promising Endometrial Cancer Treatment
MorphoSys AG today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for tulmimetostat, an innovative dual inhibitor targeting EZH2 and EZH1. This breakthro ...
CyGenica Receives Orphan Drug Designation for Glioblastoma Multiforme (GBM) Treatment
CyGenica Limited, a dynamic biotech startup, has achieved a significant milestone in the fight against Glioblastoma Multiforme (GBM), an aggressive form of brain cancer. Dr. Nusrat J M Sanghamitra, Co ...
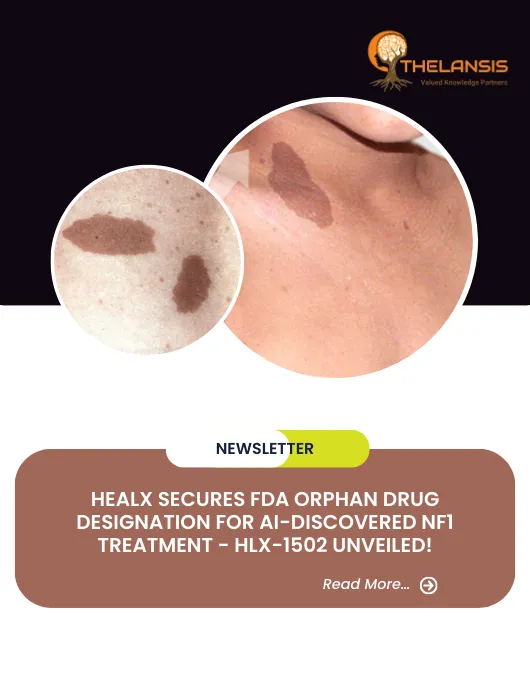
Healx Secures FDA Orphan Drug Designation for AI-Discovered NF1 Treatment – HLX-1502 Unveiled!
Healx, a cutting-edge techbio company driven by AI innovation and patient-centricity, has achieved Orphan Drug Designation from the US Food and Drug Administration (FDA) for its groundbreaking AI-driv ...

