
FDA Grants Fast Track Designation to MorphoSys AG’s Promising Endometrial Cancer Treatment
MorphoSys AG today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for tulmimetostat, an innovative dual inhibitor targeting EZH2 and EZH1. This breakthro ...
CyGenica Receives Orphan Drug Designation for Glioblastoma Multiforme (GBM) Treatment
CyGenica Limited, a dynamic biotech startup, has achieved a significant milestone in the fight against Glioblastoma Multiforme (GBM), an aggressive form of brain cancer. Dr. Nusrat J M Sanghamitra, Co ...
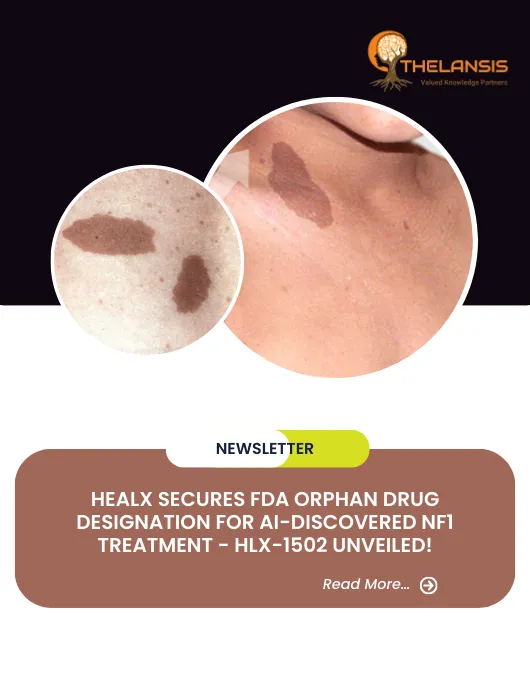
Healx Secures FDA Orphan Drug Designation for AI-Discovered NF1 Treatment – HLX-1502 Unveiled!
Healx, a cutting-edge techbio company driven by AI innovation and patient-centricity, has achieved Orphan Drug Designation from the US Food and Drug Administration (FDA) for its groundbreaking AI-driv ...

INOVIO’s INO-3107 Granted FDA Breakthrough Therapy Designation for RRP Treatment
INOVIO Pharmaceuticals, a leading biotechnology company, has received the FDA's Breakthrough Therapy designation for INO-3107. This DNA medicine shows promise in treating Recurrent Respiratory Papillo ...

