Jan 30 2023
/
Nanoscope Therapeutics’ MCO-010 Receives Fast Track Designation from FDA for Stargardt Disease Treatment
Nanoscope Therapeutics, a clinical-stage biotech company specializing in gene therapies for retinal disorders, announced that the US FDA has granted Fast Track Designation to MCO-010. an ambient-light activatable Multi-Characteristic Opsin (MCO) optogenetic monotherapy to restore vision in blind patients for the treatment of Stargardt disease to improve visual function. Enrollment in Phase 2 open-label STARLIGHT clinical trial (NCT05417126) of MCO-010 was completed in September 2022.
Publish Date: 30-01-2023 Source: Nanoscope Therapeutics Inc.
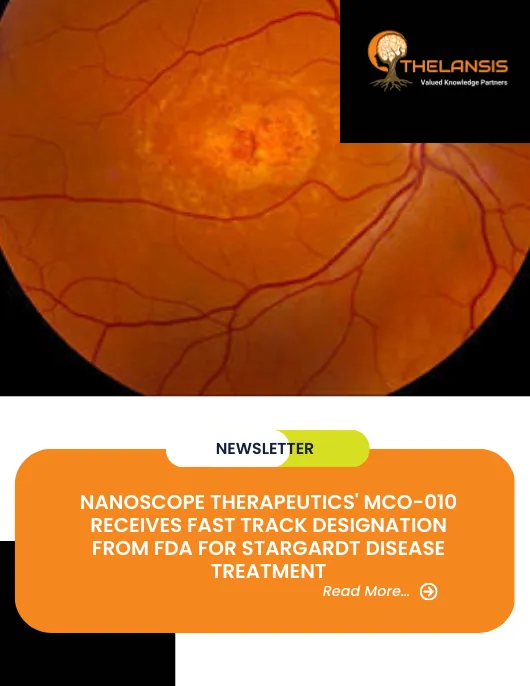
Stargardt disease is a common form of juvenile hereditary macular degeneration caused by mutations in the ABCA4 gene. It is characterized by small yellowish round spots around the macula near the retinal pigment epithelium. The mutated ABCA4 gene produces a dysfunctional protein that cannot perform energy transport between photoreceptor cells in the retina. Without energy, the photoreceptor cells degenerate, leading to loss of vision. The onset of SD is usually within the first ten to fifteen years of an individual’s life. It causes vision loss in the range of 20/50 to 20/200 on a standard eye chart. Electrophysiological assessment, including multifocal, pattern, and full-field electroretinography, can be helpful in confirming the diagnosis of SD. Imaging techniques providing an enhanced assessment of retinal architecture have also been proven valuable in diagnosis, monitoring, and probing the pathogenesis of SD.
- The annual incidence rate of Stargardt disease ranges between 15 to 25 cases per 100,000 in the USA.
However, the current Stargardt disease treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (LBS-008, Emixustat, etc.) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Stargardt disease treatment. The key companies in the advanced development stage are Belite Bio, Inc, Kubota Vision Inc., etc., targeting SD.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Stargardt disease to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com - Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com
Related posts:
- FDA grants AOC 1001 Fast Track Designation to treat Myotonic Dystrophy Type 1 (DM1).
- Prestige Biopharma’s PBP1510 Granted Fast Track Designation by FDA for Advanced Pancreatic Cancer Treatment
- Verismo Therapeutics Receives Fast Track Designation from FDA for SynKIR-110, a Novel CAR T Therapy for Mesothelioma Treatment
- FDA Fast Tracks FELIQS’ FLQ-101 for Preventing Retinopathy of Prematurity (ROP)

