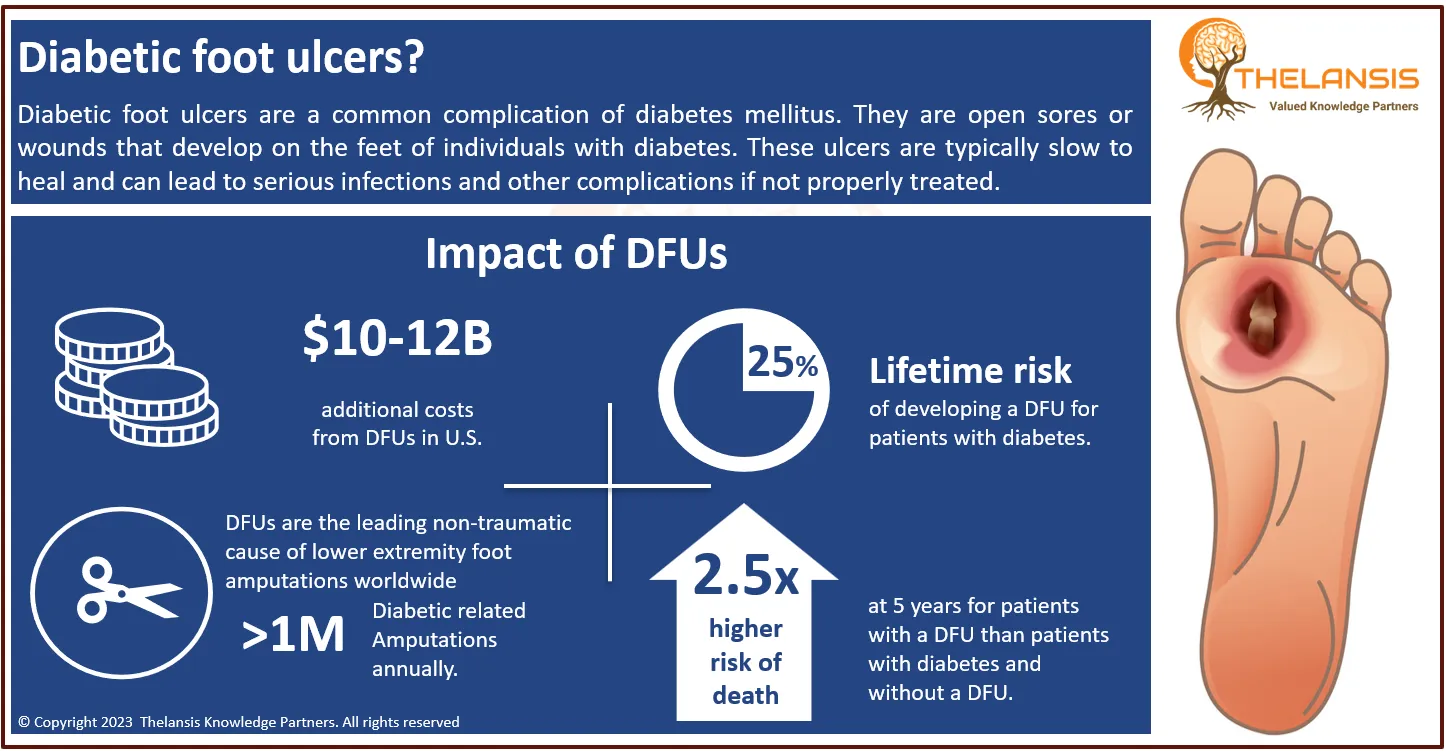
Moderna's mRNA-1345 Receives Breakthrough Designation from FDA for Respiratory Syncytial Virus (RSV) Prevention
Moderna, a biotechnology company pioneering mRNA therapeutics and vaccines, announced that mRNA-1345, its investigational mRNA vaccine for respiratory syncytial virus (RSV), has received Breakthrough Therapy Designation from the U.S. FDA. The designation was based on the success of the Phase 3 trial, “ConquerRSV,” which showed positive results in preventing RSV-associated lower respiratory tract disease (RSV-LRTD) in adults 60 years and older.
Publish Date: 30-01-2023 Source: Moderna, Inc.
Respiratory syncytial virus (RSV) is one of the most common causes of bronchiolitis and pneumonia in children under one year in the United States. During the RSV season, it is a common reason for young infants to be hospitalized. RSV can also cause serious respiratory illness in elderly people. Most healthy people recover from RSV infection within 1 to 2 weeks. However, the infection can be serious in a few people, usually those who are at higher risk for some reason. By the age of two, almost all children have been infected with the virus. Most children do not suffer from a serious illness. RSV infections can be found all over the world. RSV is an under-recognized cause of health deterioration, particularly in the frail elderly. Infection rates generally rise in late autumn and early winter, causing infant bronchiolitis, common colds in adults, and insidious respiratory illness in the elderly.
- RSV plays a causative role in an estimated 40% to 75% of cases of hospitalized bronchiolitis, 15% to 40% of cases of childhood pneumonia, and 6% to 15% of cases of croup.
However, the current Respiratory Syncytial Virus (RSV) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (AK0529, MEDI8897, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Respiratory Syncytial Virus (RSV) treatment. The key companies in the advanced development stage are Ark Biosciences Inc., MedImmune LLC, etc., targeting RSV.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Respiratory Syncytial Virus (RSV) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com