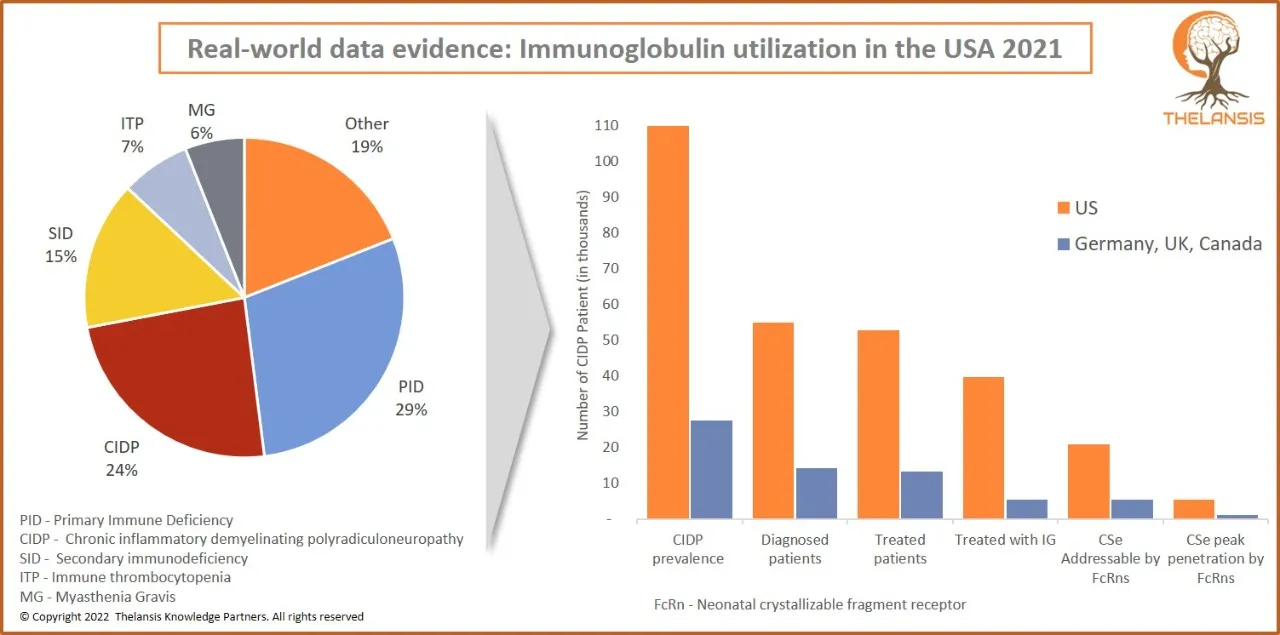
Real-world data evidence: Immunoglobulin utilization in the USA 2021
[vc_row][vc_column][vc_custom_heading text="Real-world data evidence: Immunoglobulin utilization in the USA 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][ ...
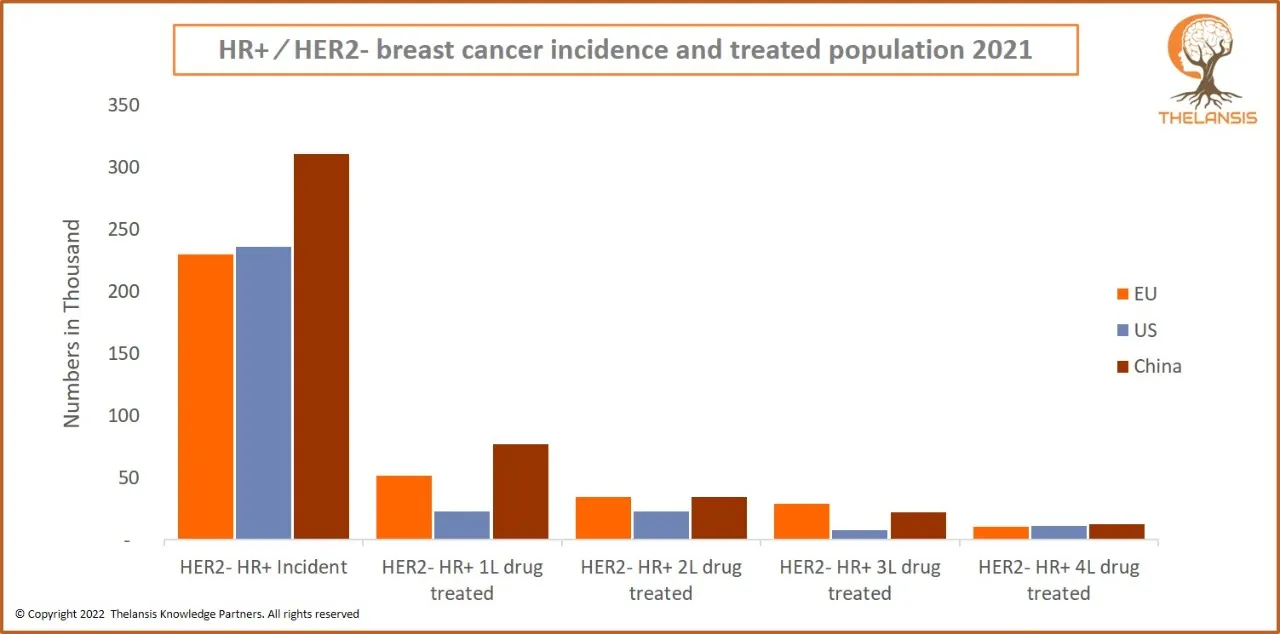
HR+/HER2- Breast Cancer Incidence and Treated Population 2021
[vc_row][vc_column][vc_custom_heading text="HR+/HER2- Breast Cancer Incidence and Treated Population 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row] ...
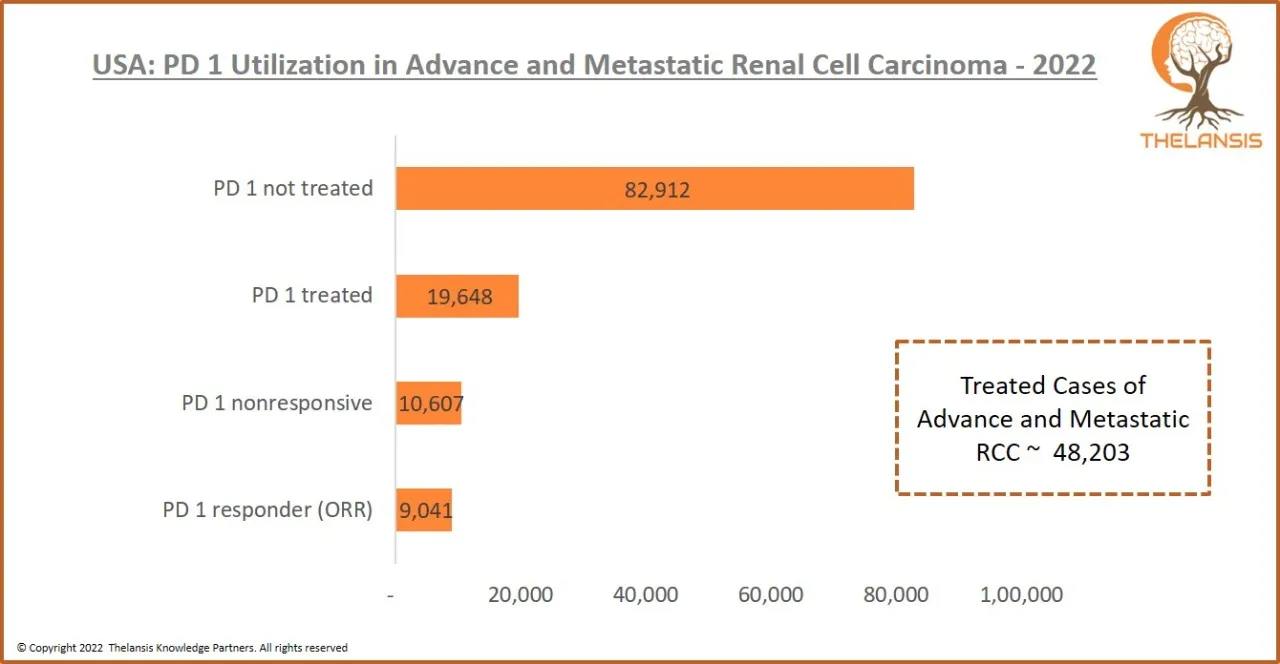
USA: PD 1 Utilization in Advance and Metastatic Renal Cell Carcinoma-2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Renal Cell Carcinoma-2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_ ...
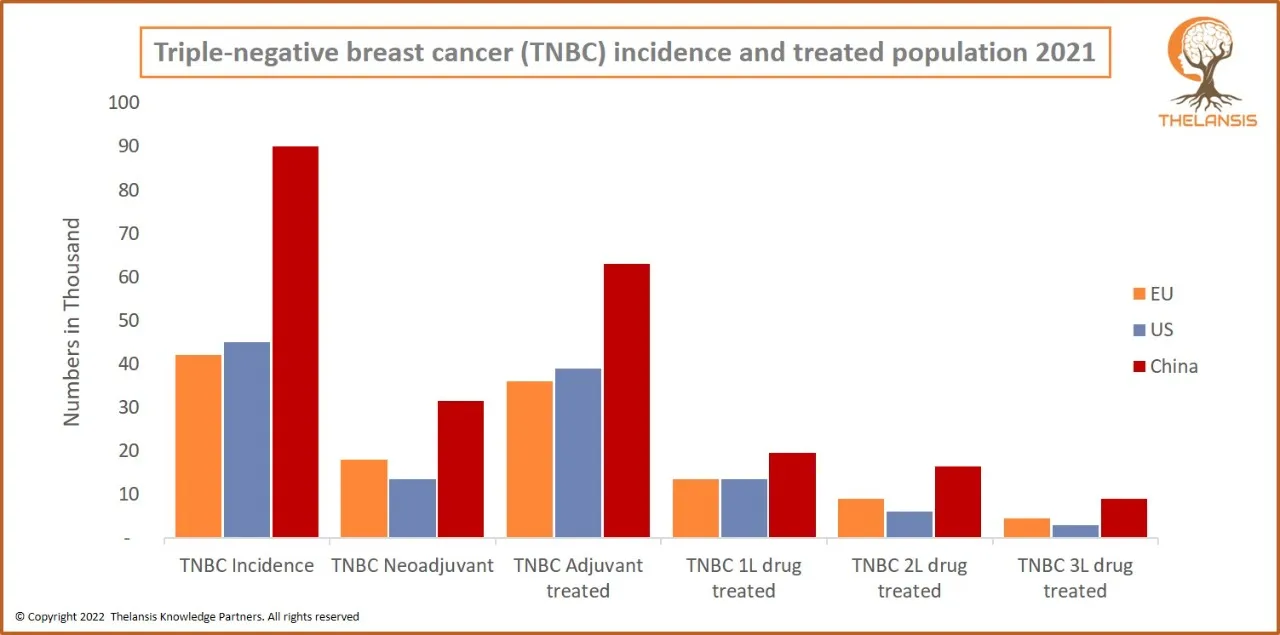
Triple-Negative Breast Cancer (TNBC) Incidence and Treated Population 2021
[vc_row][vc_column][vc_custom_heading text="Triple-Negative Breast Cancer (TNBC) Incidence and Treated Population 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc ...
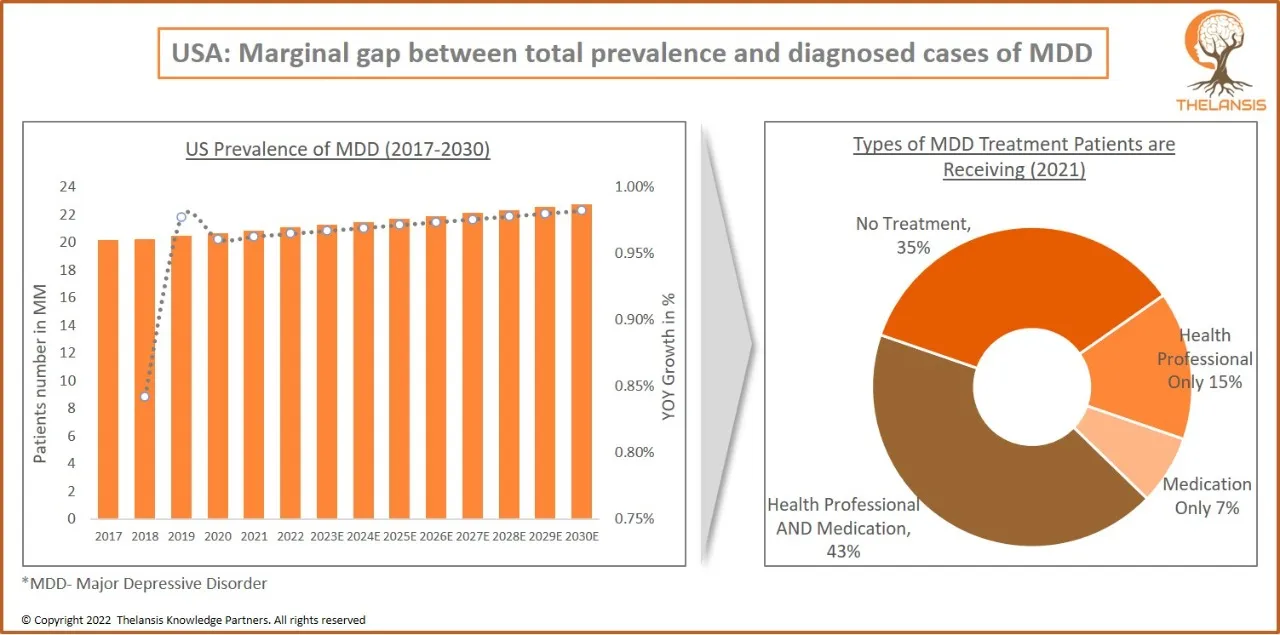
USA: Marginal gap between total prevalence and diagnosed cases of MDD
[vc_row][vc_column][vc_custom_heading text="USA: Marginal gap between total prevalence and diagnosed cases of MDD" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row] ...
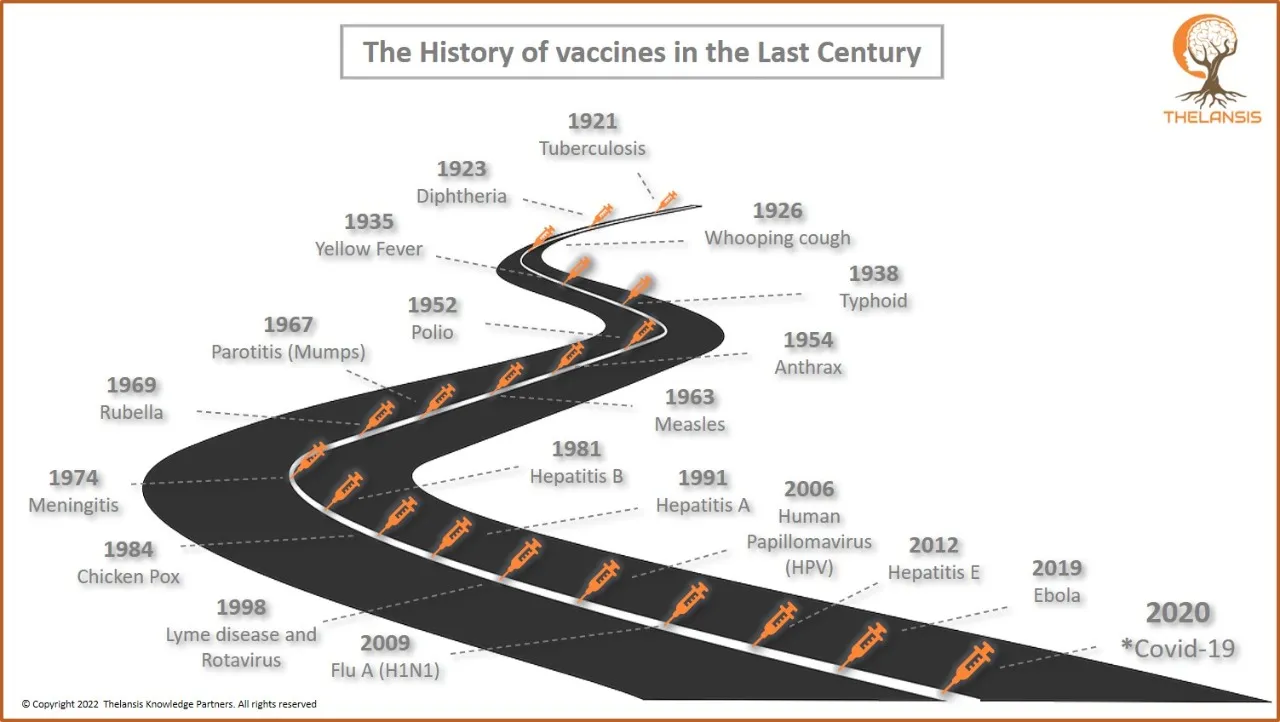
The History of vaccines in the Last Century
[vc_row][vc_column][vc_custom_heading text="The History of vaccines in the Last Century" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".v ...
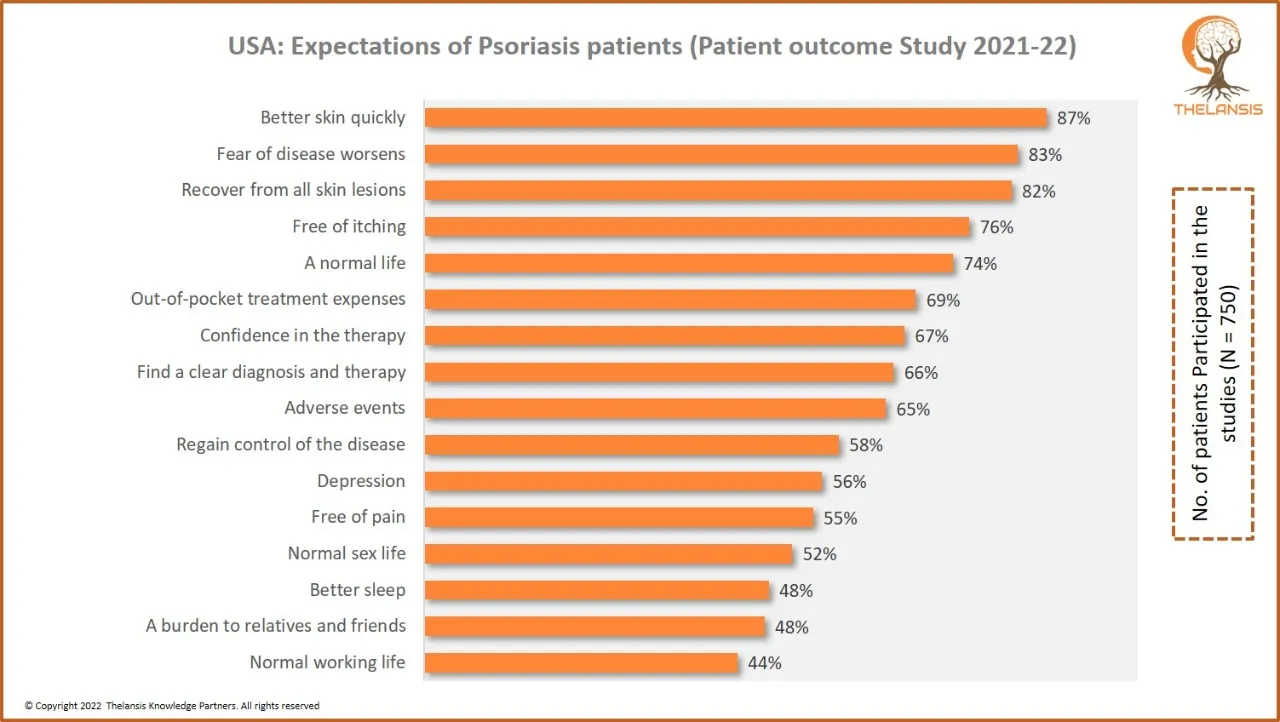
USA: Expectations of Psoriasis patients (Patient outcome study 2021-22)
[vc_row][vc_column][vc_custom_heading text="USA: Expectations of Psoriasis patients (Patient outcome study 2021-22)" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_ro ...
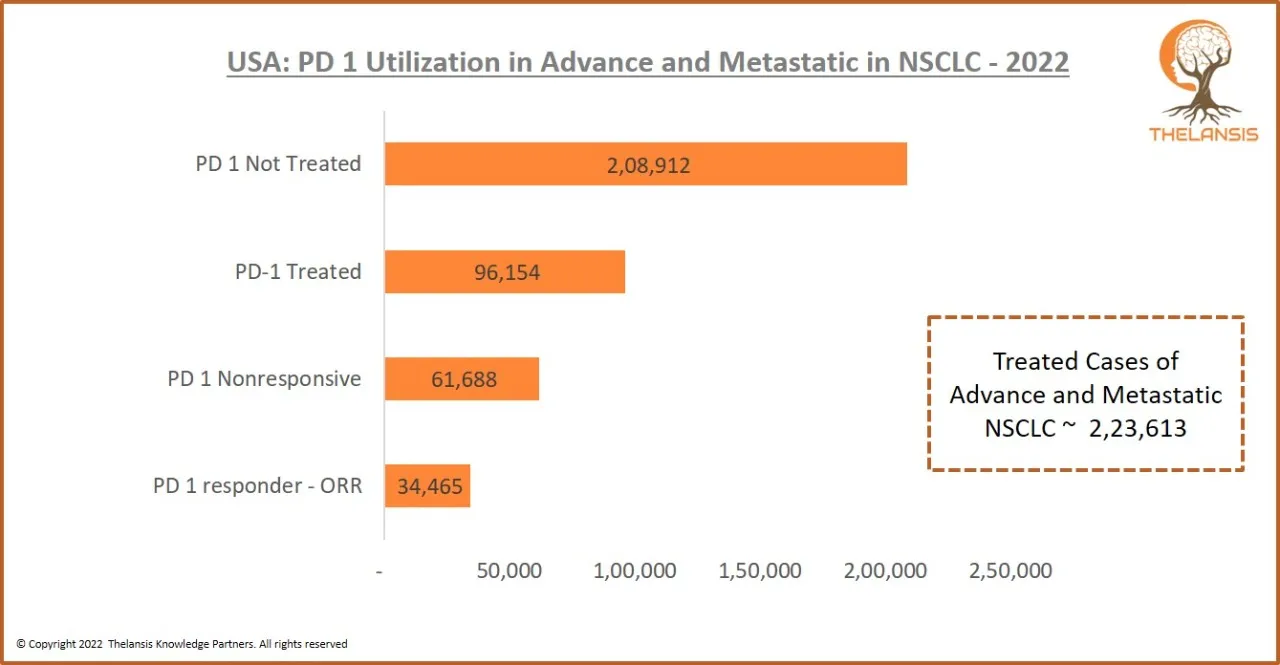
USA: PD 1 Utilization in Advance and Metastatic in NSCLC-2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic in NSCLC-2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row] ...
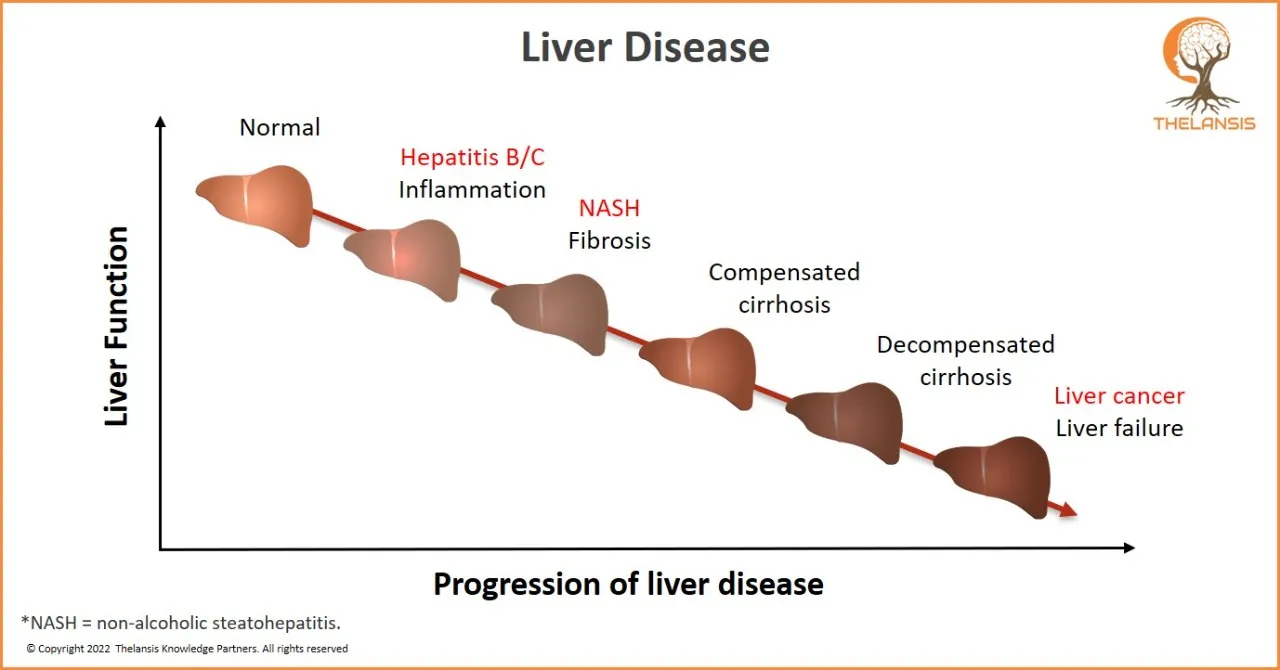
Progression of Liver Disease
[vc_row][vc_column][vc_custom_heading text="Progression of Liver Disease" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custom_165710 ...
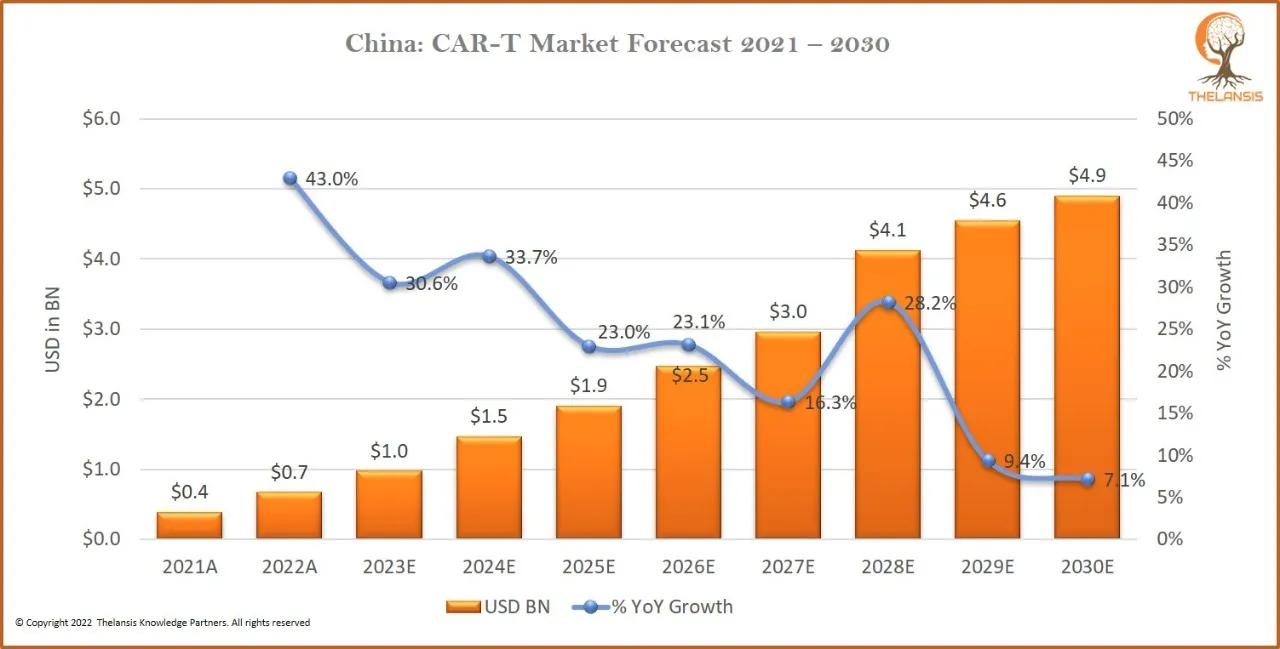
China: CAR-T Market Forecast 2021-2030
[vc_row][vc_column][vc_custom_heading text="China: CAR-T Market Forecast 2021-2030" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_cus ...

