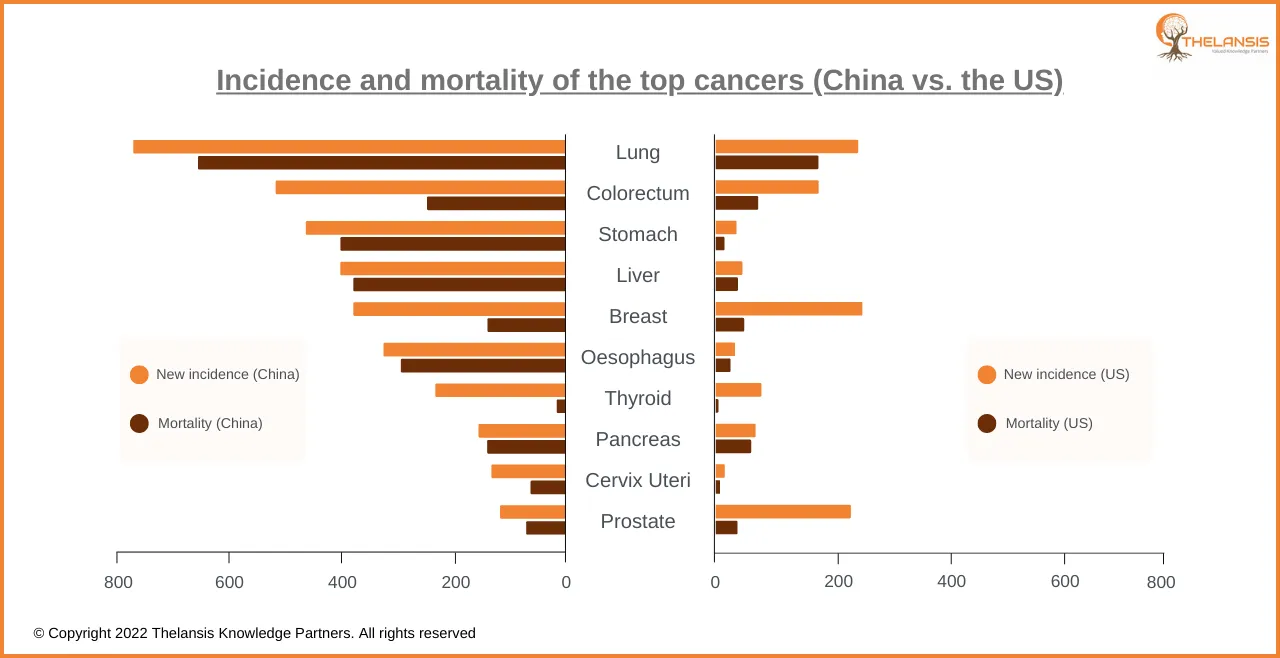
Incidence and mortality of the top cancers (China vs. the US)
[vc_row][vc_column][vc_custom_heading text="Incidence and mortality of the top cancers (China vs. the US)" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row] ...
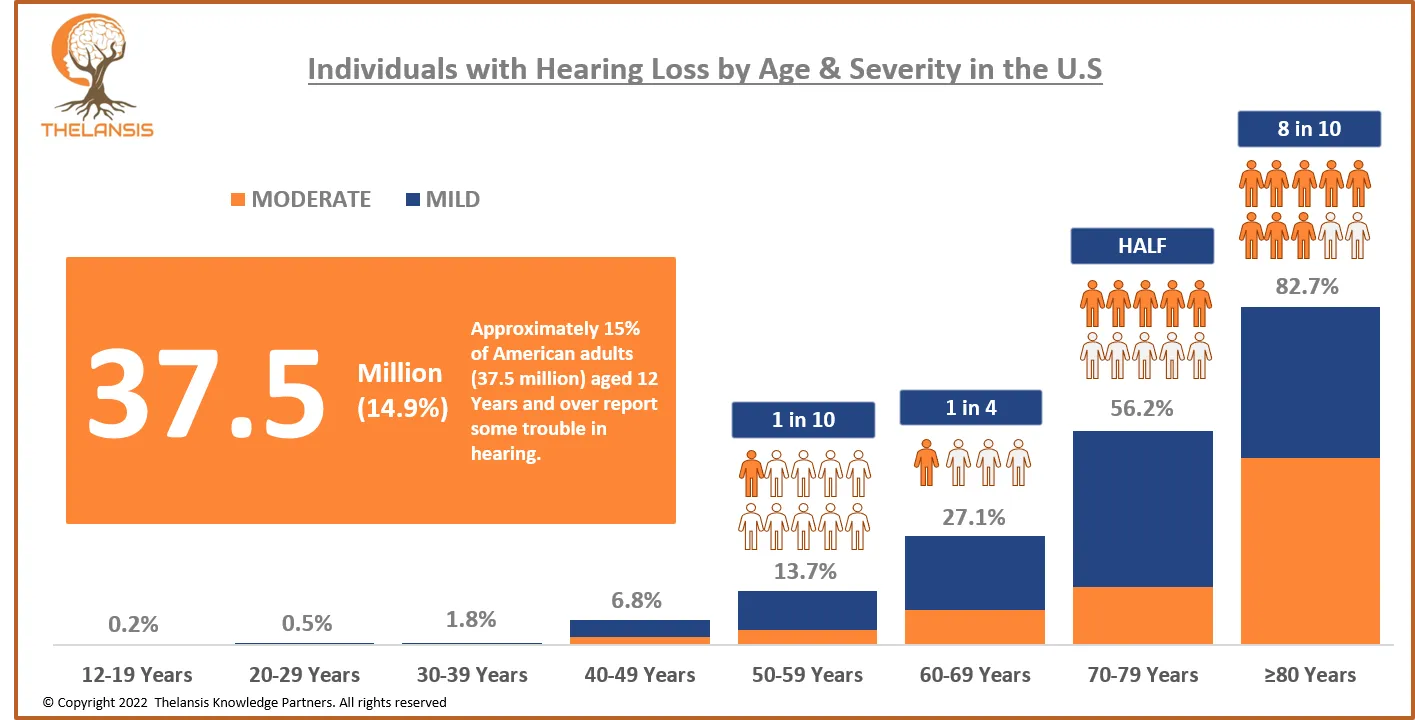
Individuals with Hearing Loss by Age & Severity in the U.S.
[vc_row][vc_column][vc_custom_heading text="Individuals with Hearing Loss by Age & Severity in the U.S." font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_ro ...
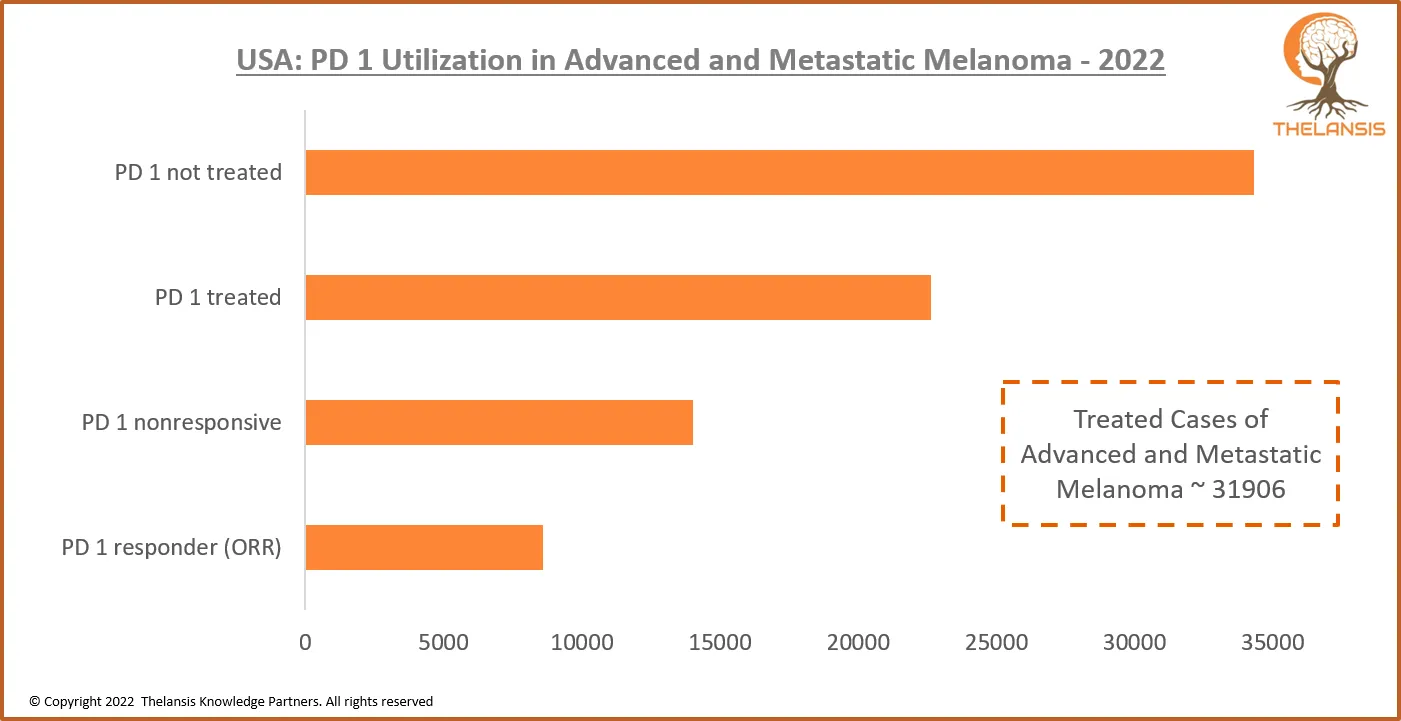
USA: PD 1 Utilization in Advanced and Metastatic Melanoma – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advanced and Metastatic Melanoma - 2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_r ...
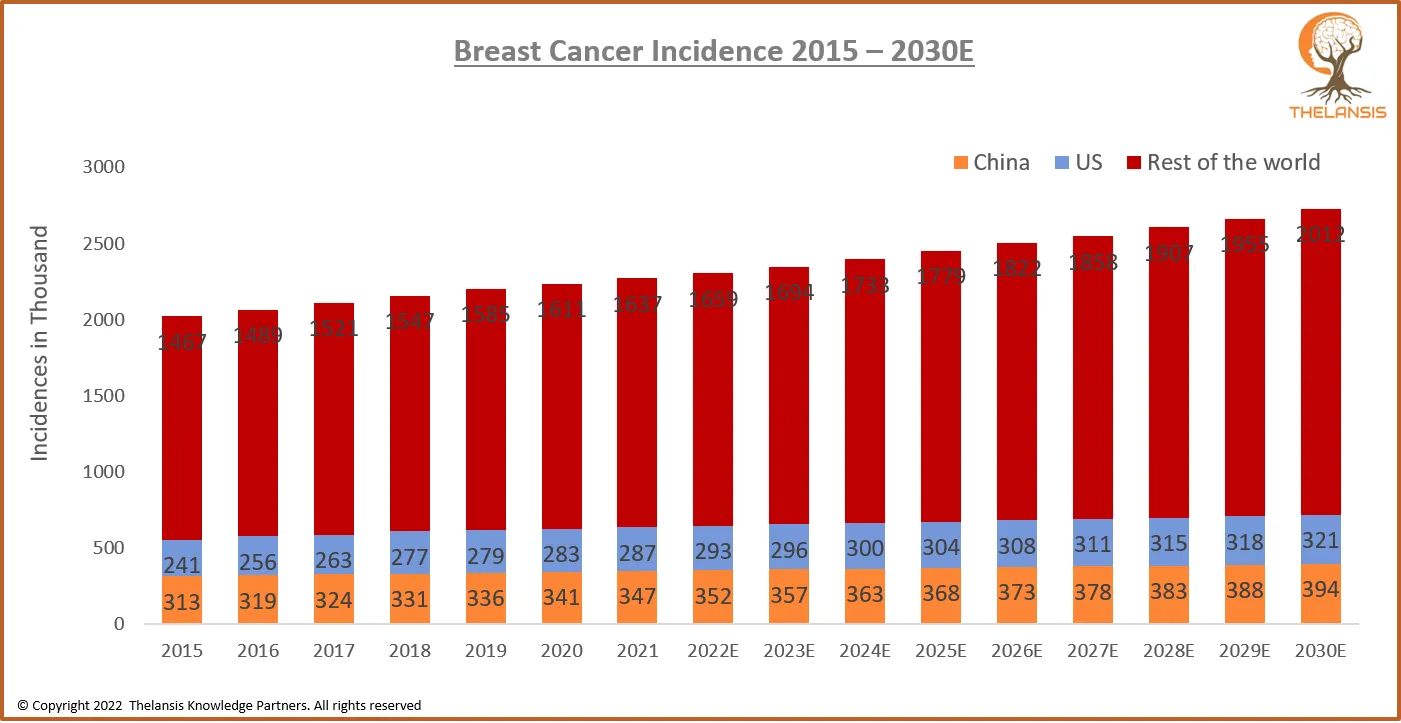
Breast Cancer Incidence 2015 – 2030E
[vc_row][vc_column][vc_custom_heading text="Breast Cancer Incidence 2015 – 2030E" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custo ...
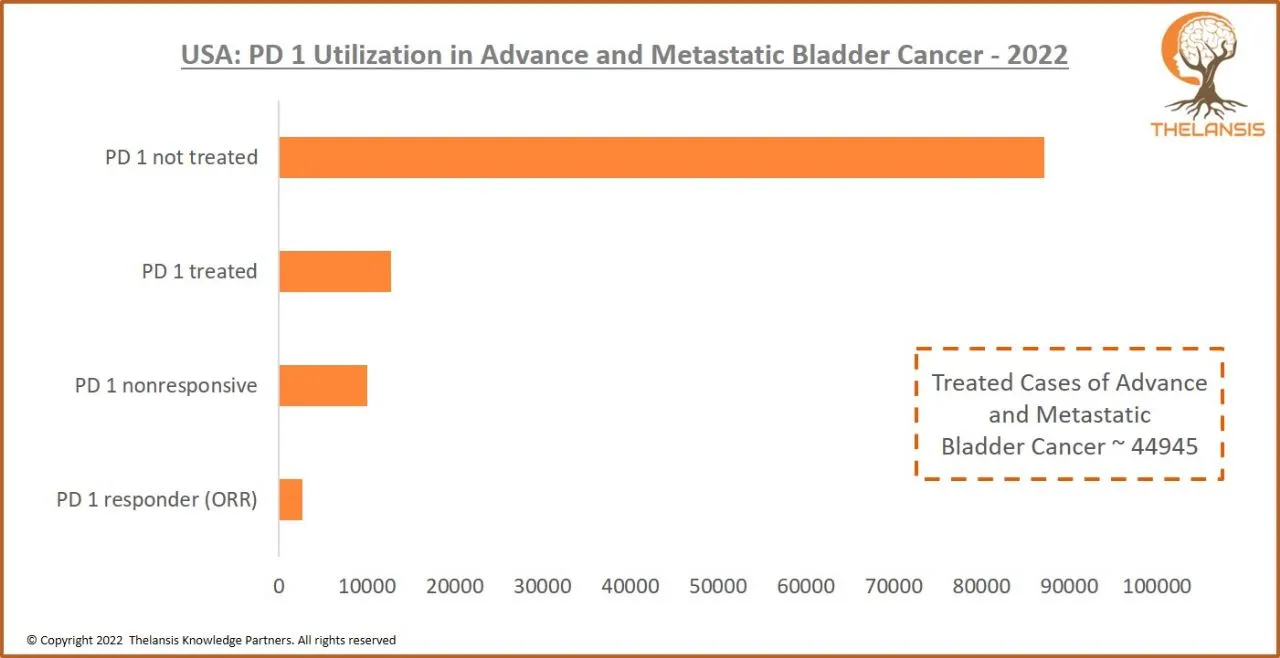
USA: PD 1 Utilization in Advance and Metastatic Bladder Cancer – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Bladder Cancer - 2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row] ...
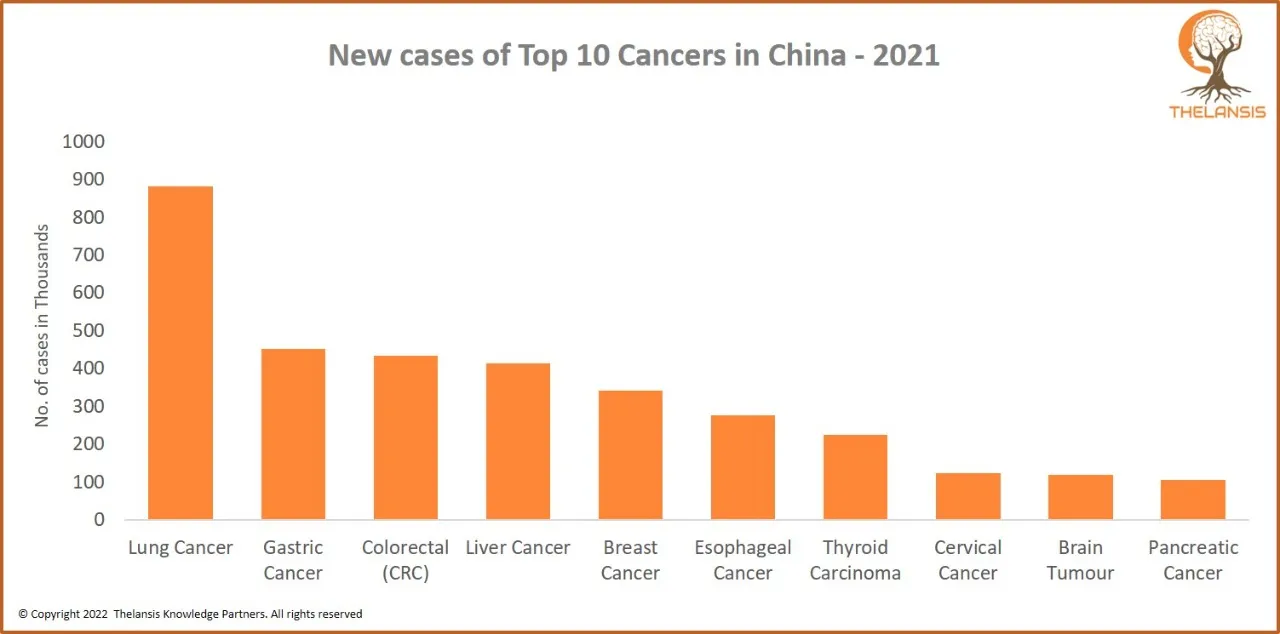
New Cases of Top 10 cancers in China – 2021
[vc_row][vc_column][vc_custom_heading text="New Cases of Top 10 cancers in China - 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".v ...
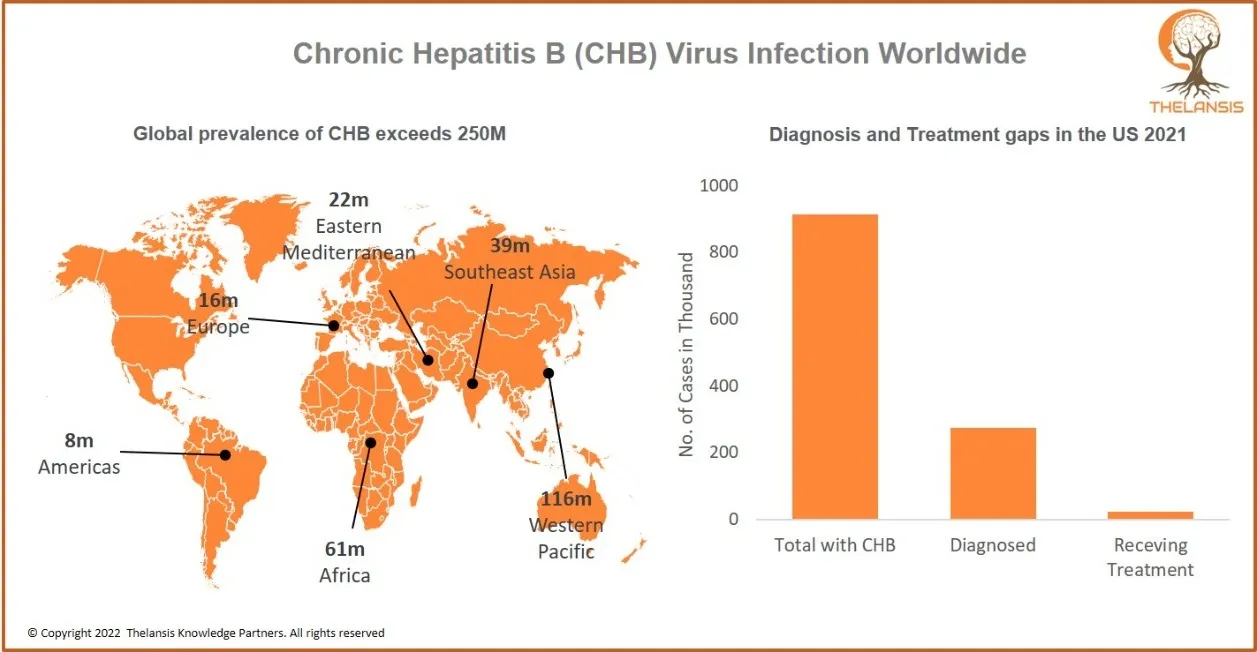
Chronic Hepatitis B (CHB) Virus Infection Worldwide
[vc_row][vc_column][vc_custom_heading text="Chronic Hepatitis B (CHB) Virus Infection Worldwide" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column ...
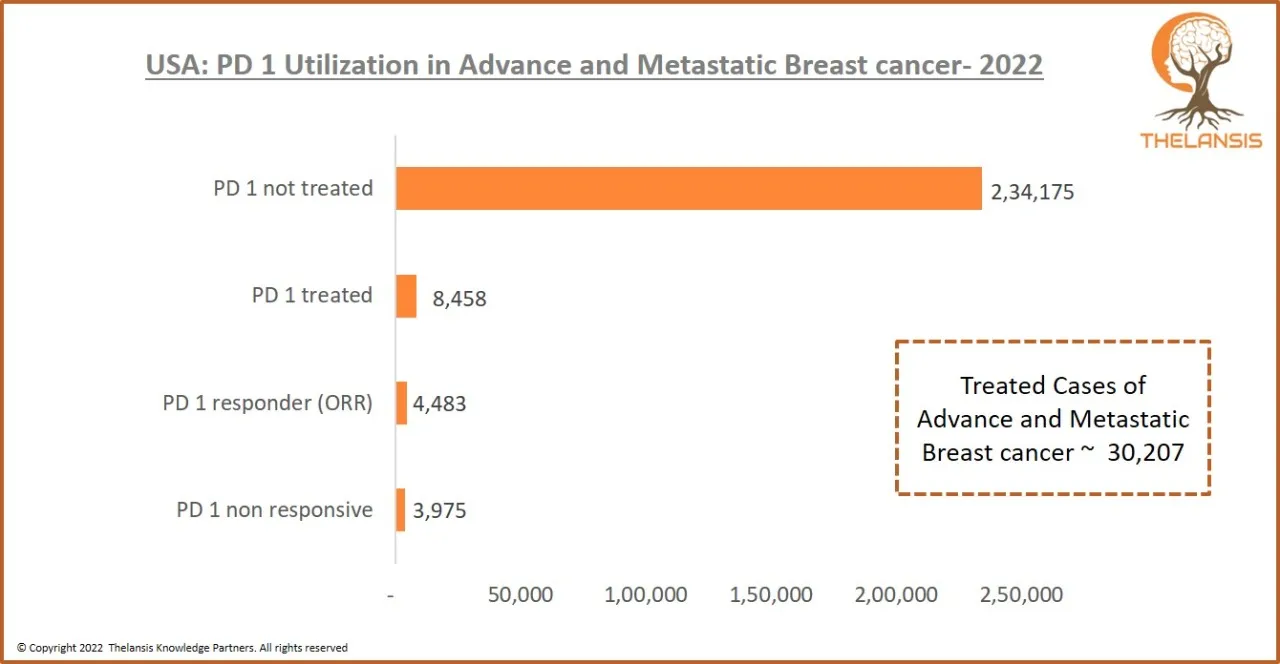
USA: PD 1 Utilization in Advance and Metastatic Breast cancer – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Breast cancer - 2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][ ...
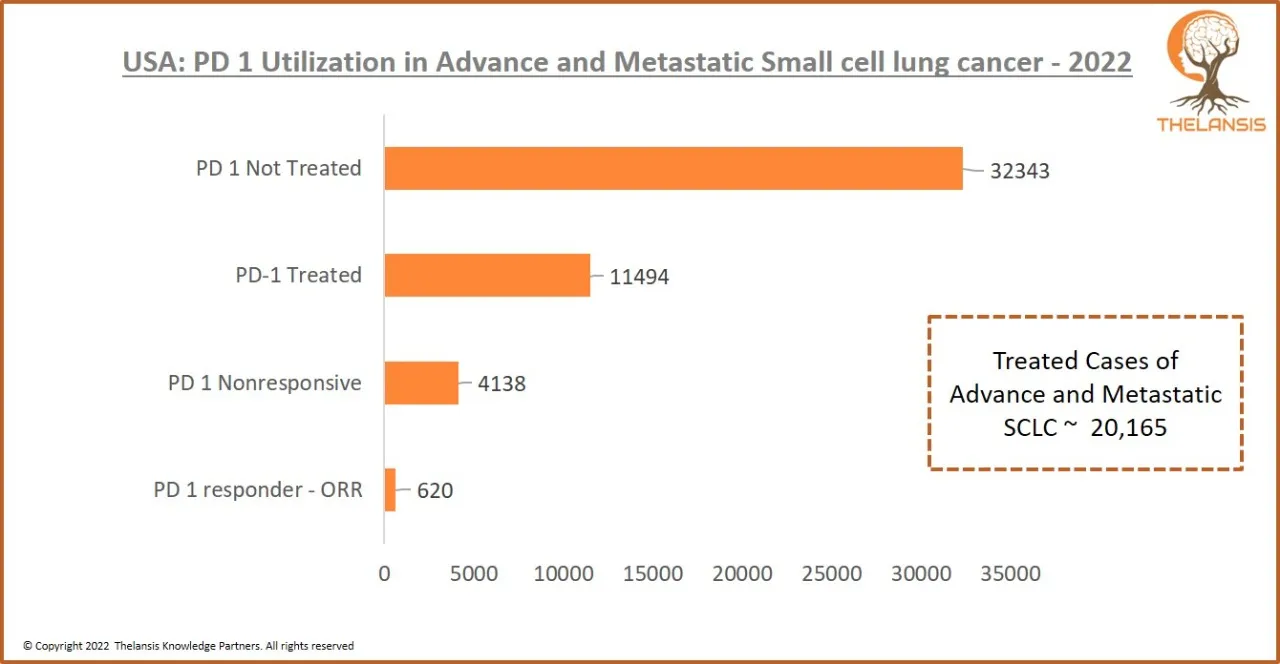
USA: PD 1 Utilization in Advance and Metastatic Small cell lung cancer – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Small cell lung cancer - 2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][ ...
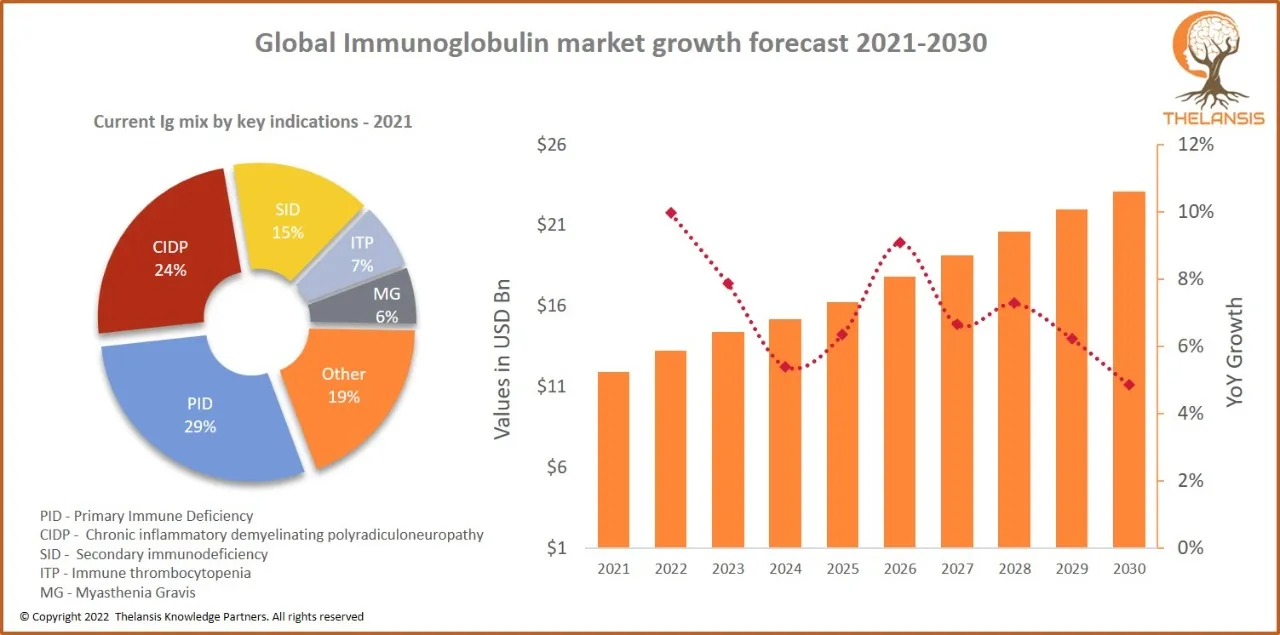
Global Immunoglobulin market growth forecast 2021-2030
[vc_row][vc_column][vc_custom_heading text="Global Immunoglobulin market growth forecast 2021-2030" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_col ...

