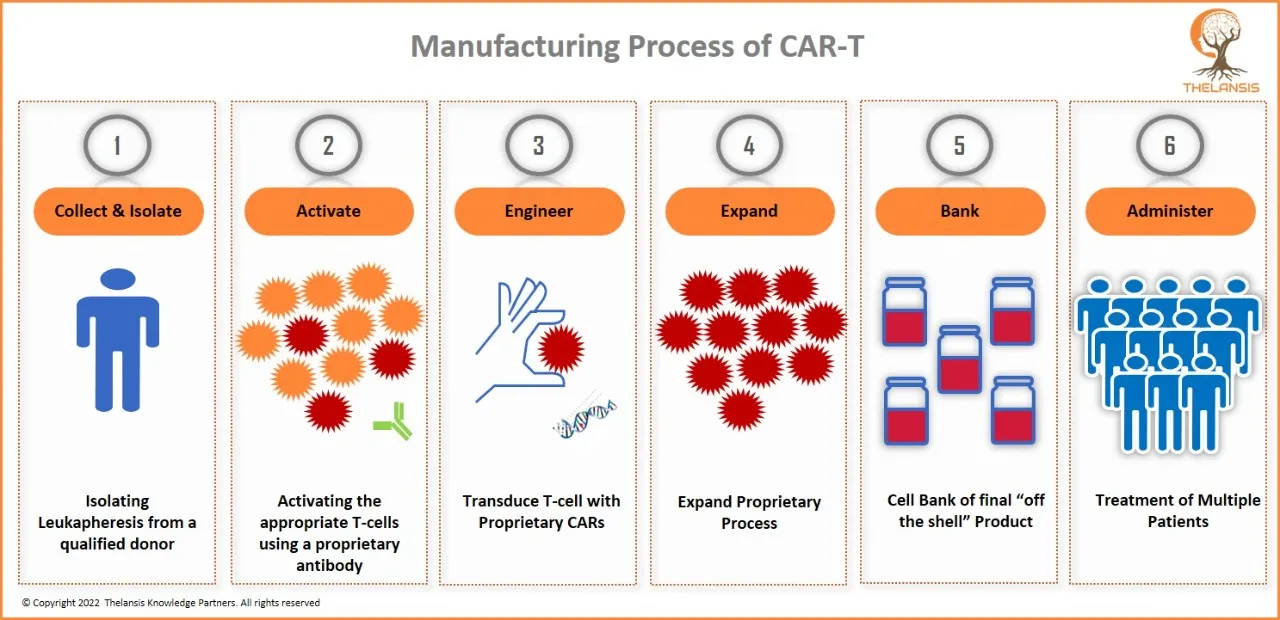
Manufacturing Process of CAR-T
[vc_row][vc_column][vc_custom_heading text="Manufacturing Process of CAR-T" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custom_1656 ...
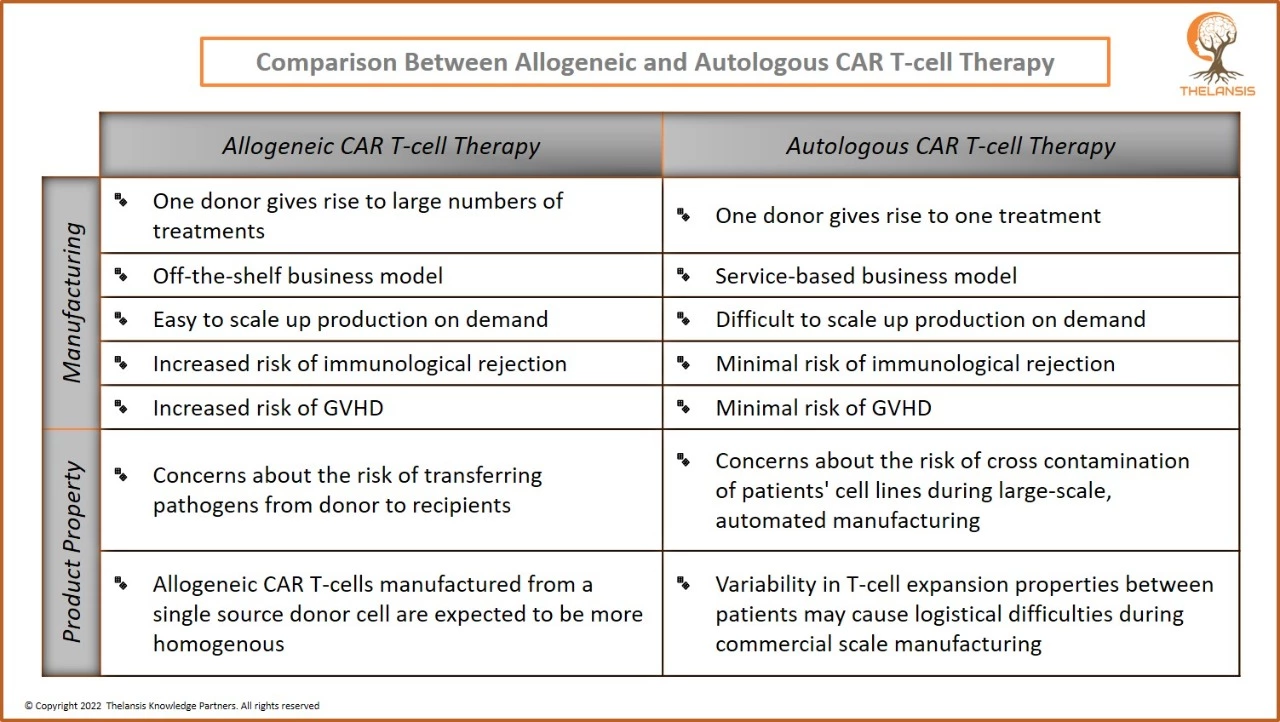
Comparison Between Allogeneic and Autologous CAR T-cell Therapy
[vc_row][vc_column][vc_column_text] Comparison Between Allogeneic and Autologous CAR T-cell Therapy [/vc_column_text][/vc_column][/vc_row][vc_row][vc_column css=".vc_custom_1656416797987{margin-top: 2 ...
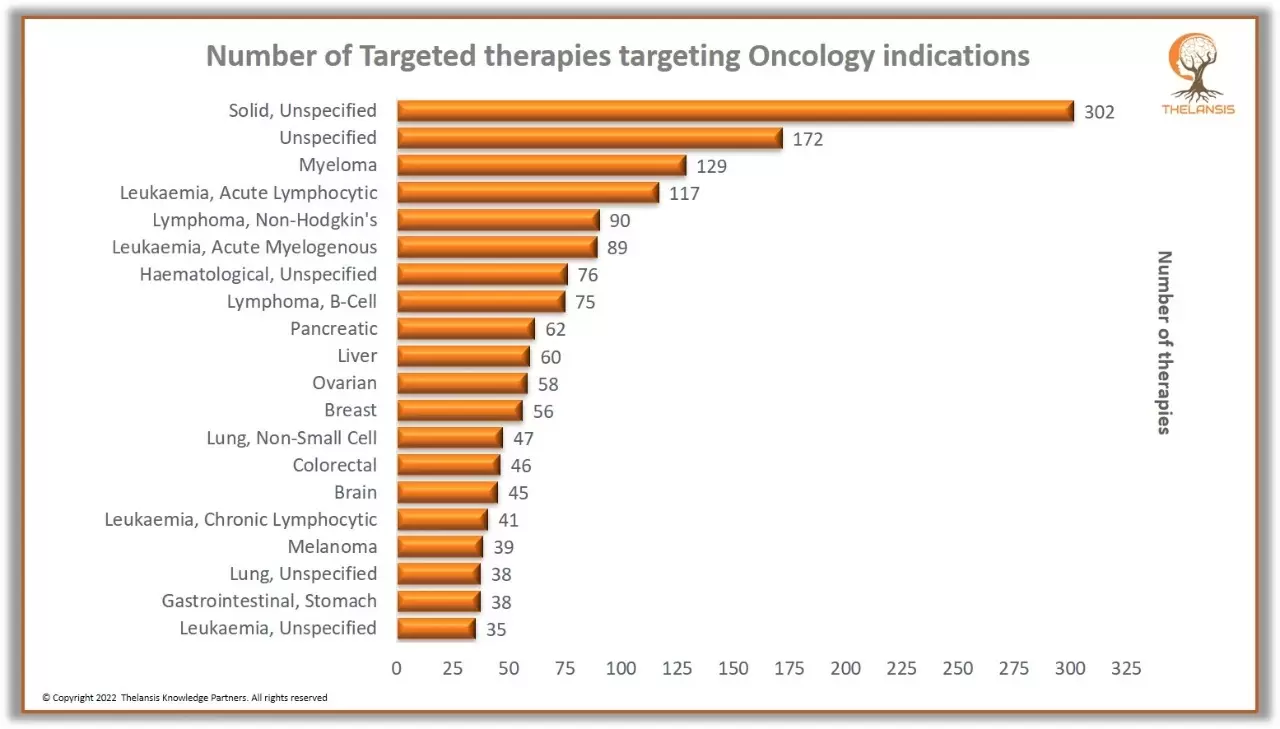
Number of Targeted therapies targeting Oncology indications
Number of Targeted therapies targeting Oncology indications
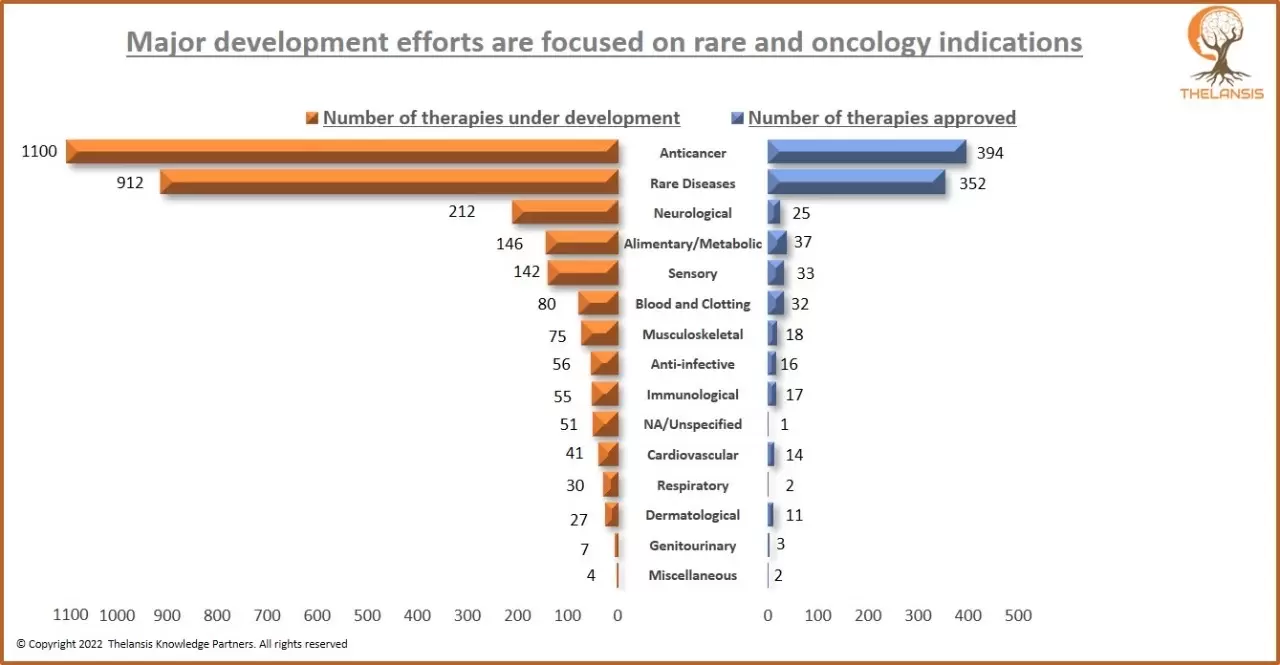
Major development efforts are focused on rare and oncology indications
Major development efforts are focused on rare and oncology indications
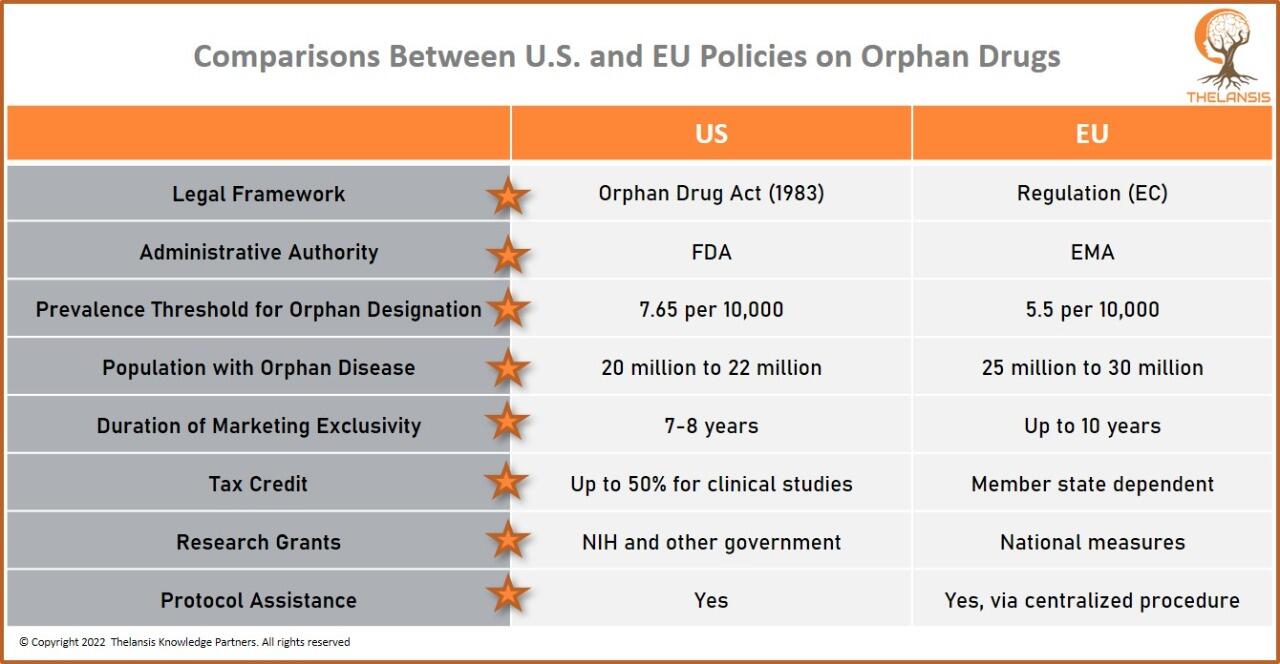
Comparisons Between U.S. and EU Policies on Orphan Drugs
Comparisons Between U.S. and EU Policies on Orphan Drugs
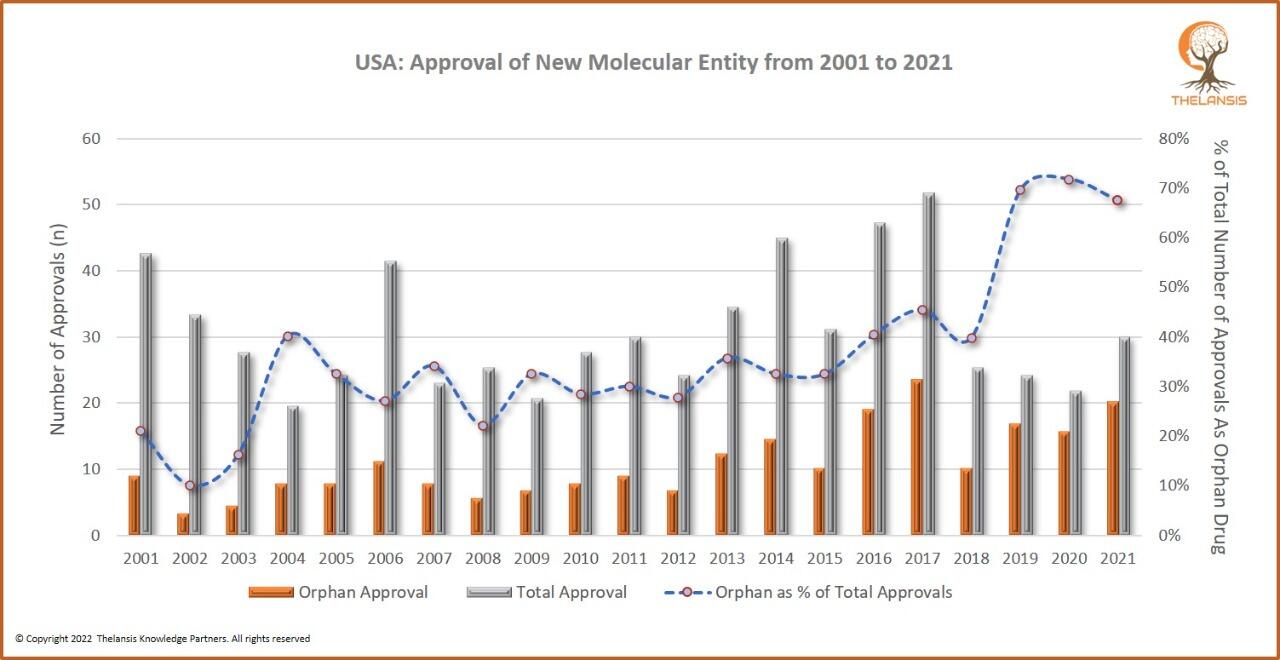
USA: Approval of New Molecular Entity from 2001 to 2021
USA: Approval of New Molecular Entity from 2001 to 2021
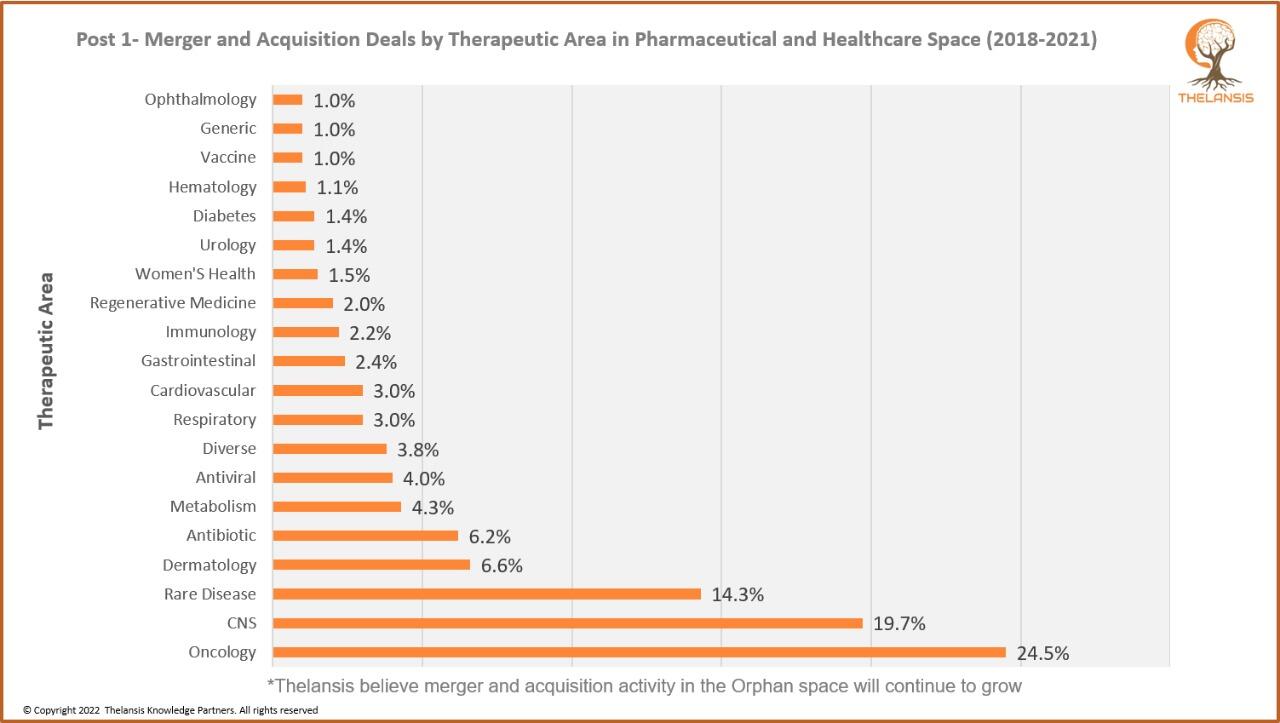
Merger and Acquisition Deals by Therapeutic Area in Pharmaceutical and Healthcare Space (2018-2021)
Merger and Acquisition Deals by Therapeutic Area in Pharmaceutical and Healthcare Space (2018-2021)

Pulmonary arterial hypertension (PAH) Thelansis comments
[vc_row][vc_column][vc_single_image image="4182" img_size="280x400" onclick="custom_link" title="Pulmonary arterial hypertension (PAH) Thelansis comments" link="https://thelansis.com/wp-content/upload ...
Biomarkers in rare diseases
Discovery of biomarkers in rare diseases: innovative approaches by predictive and personalized medicine Rare disease (or Orphan disease) is a collective terminology to define a group of conditions wit ...