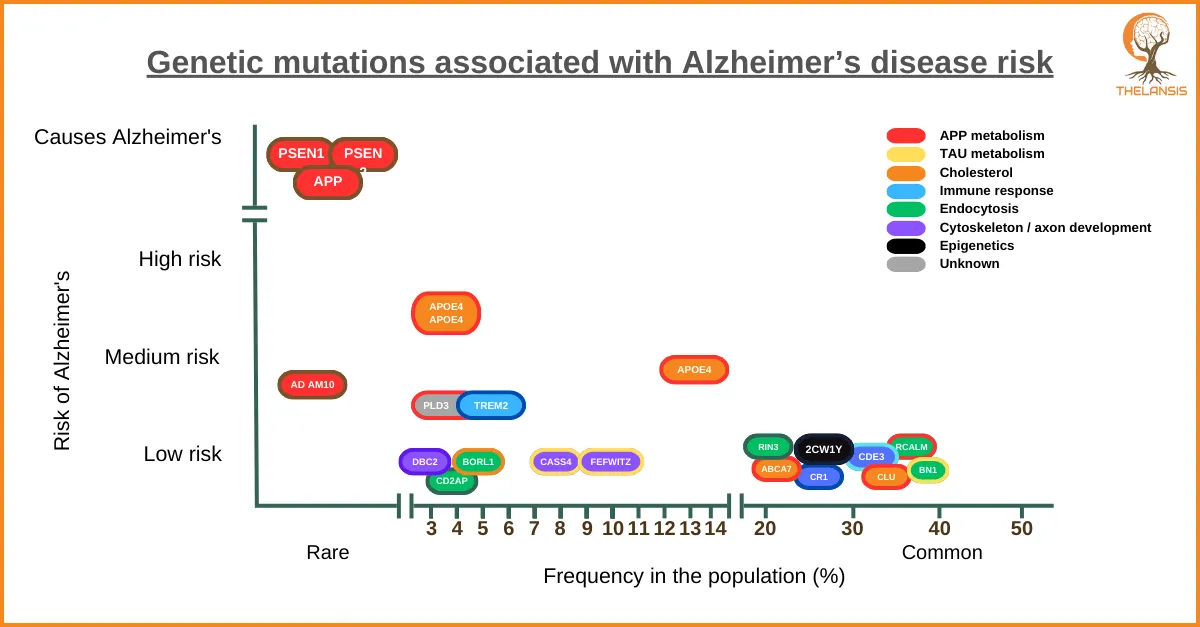
Genetic mutations associated with Alzheimer’s disease risk
[vc_row][vc_column][vc_custom_heading text="Genetic mutations associated with Alzheimer’s disease risk" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc ...

FDA Approves Apellis’ SYFOVRE™ (pegcetacoplan injection) for Geographic Atrophy (GA)
Apellis Pharmaceuticals, Inc. announced today that SYFOVRE™ (pegcetacoplan injection) has been approved by the United States Food and Drug Administration (FDA) for the treatment of geographic atrophy ...
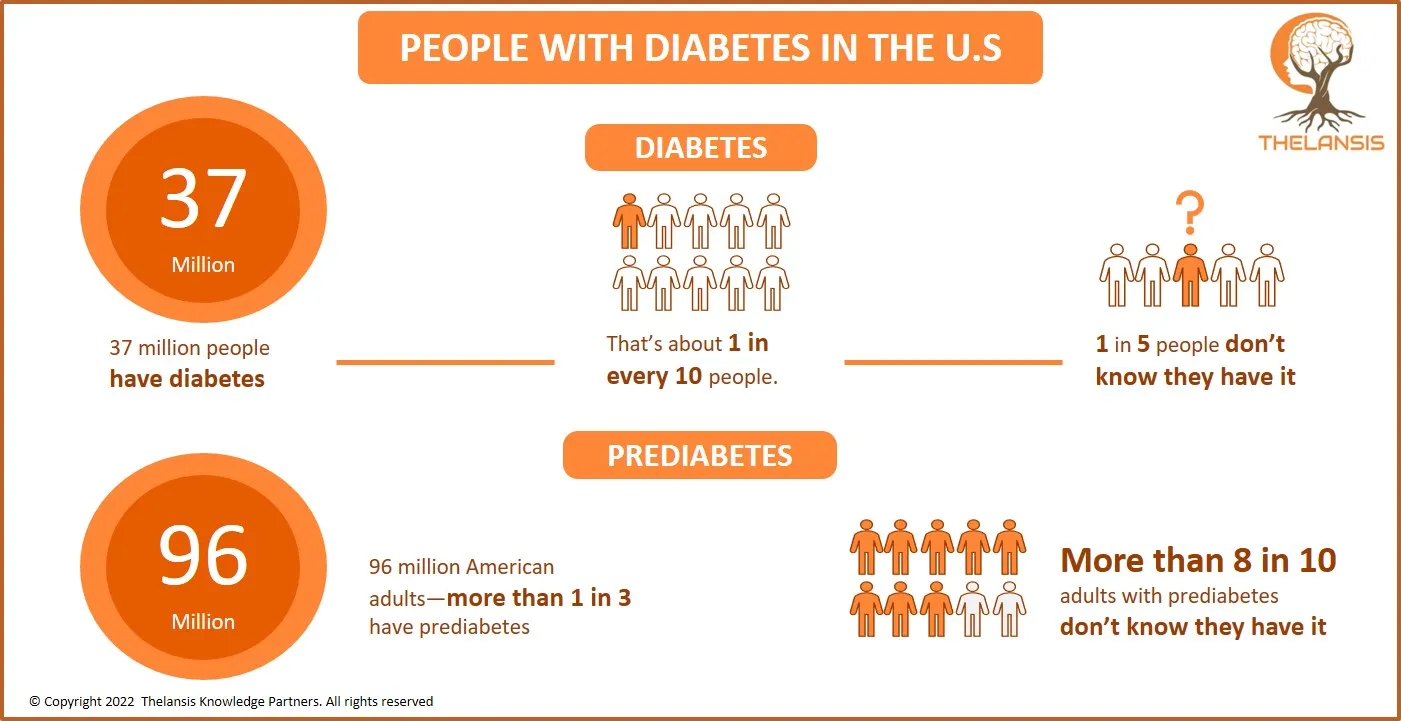
People with Diabetes in the U.S
[vc_row][vc_column][vc_custom_heading text="People with Diabetes in the U.S" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custom_166 ...
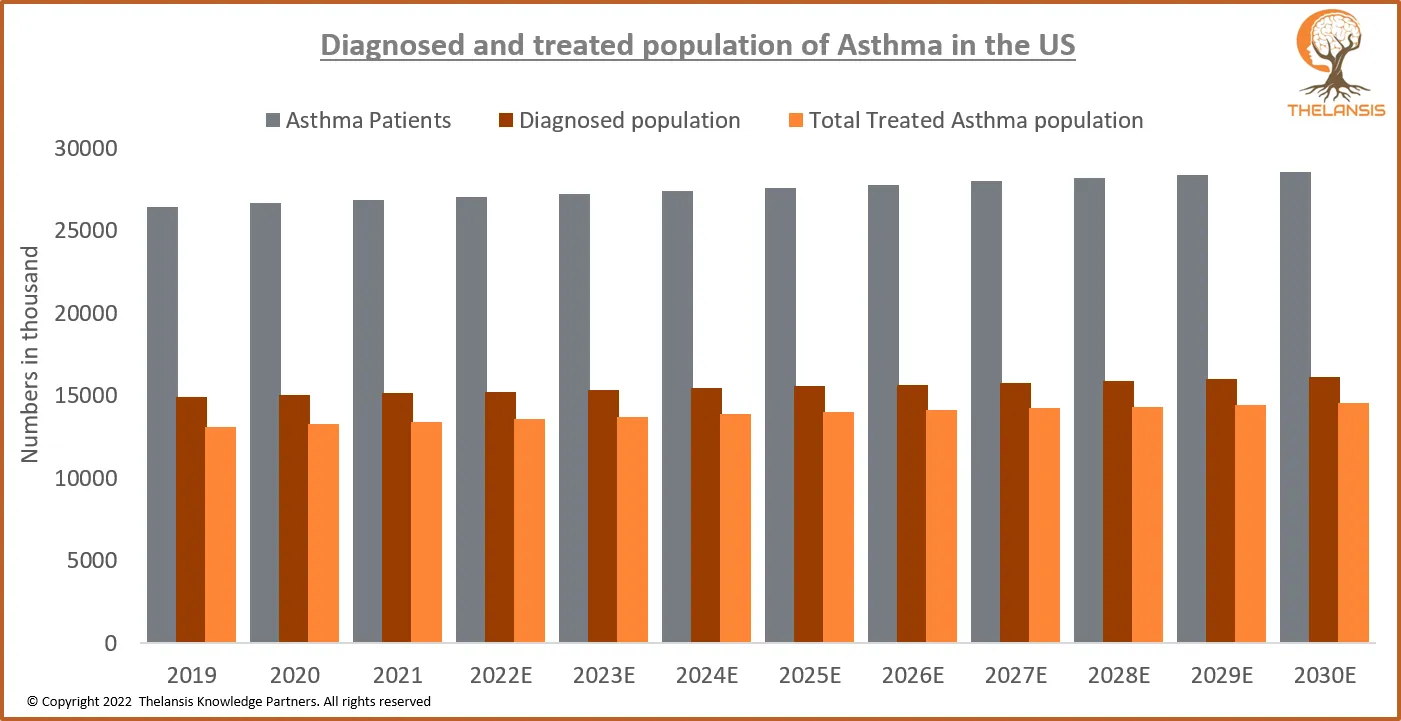
Diagnosed and Treated Population of Asthma in the US
[vc_row][vc_column][vc_custom_heading text="Diagnosed and Treated Population of Asthma in the US" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_colum ...
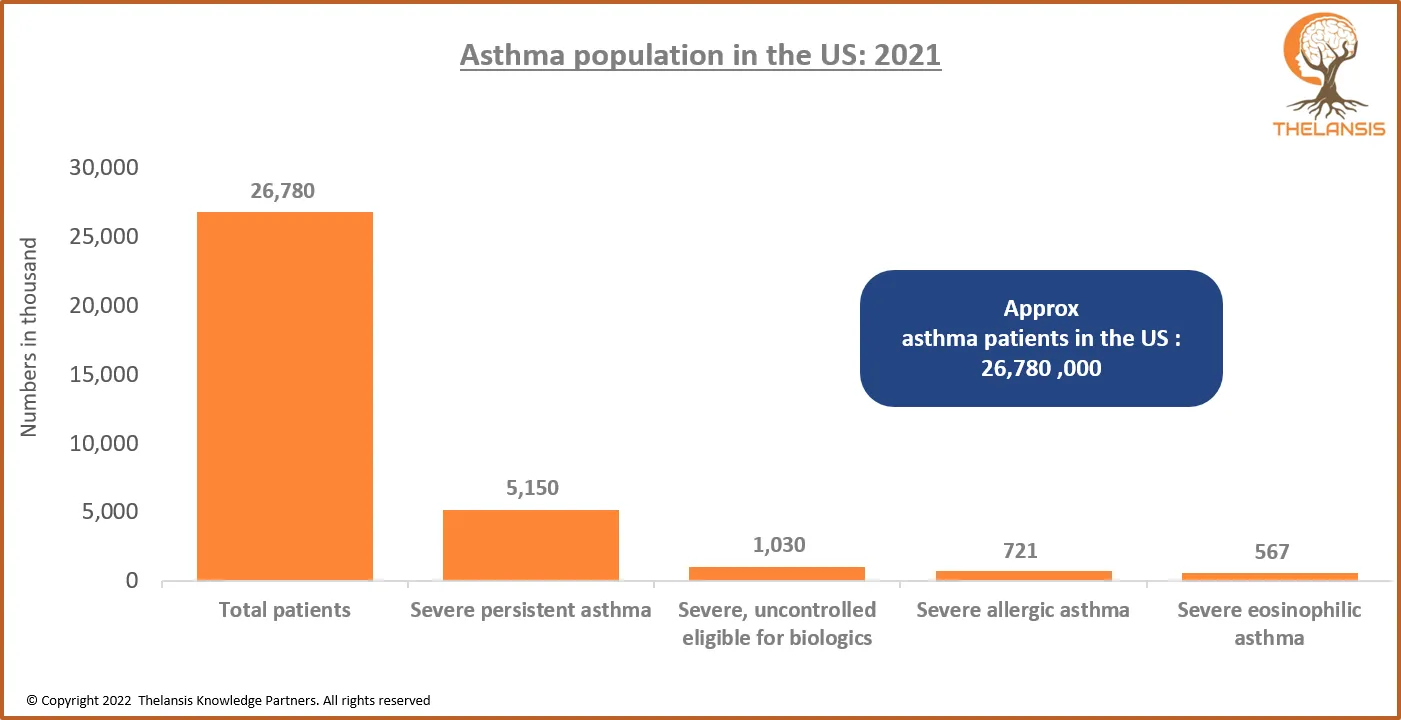
Asthma Population in the US: 2021
[vc_row][vc_column][vc_custom_heading text="Asthma Population in the US: 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_custom_1 ...
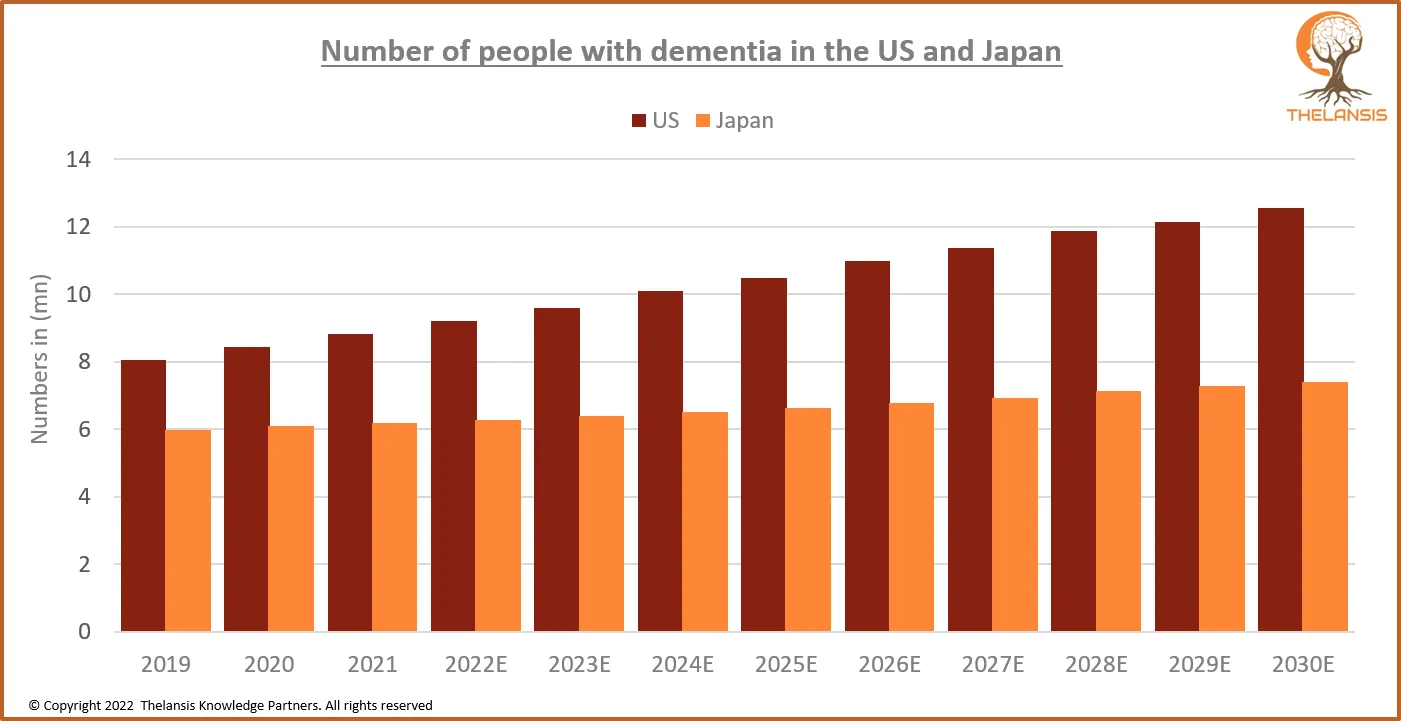
Number of people with Dementia in the US and Japan
[vc_row][vc_column][vc_custom_heading text="Number of people with Dementia in the US and Japan" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column ...
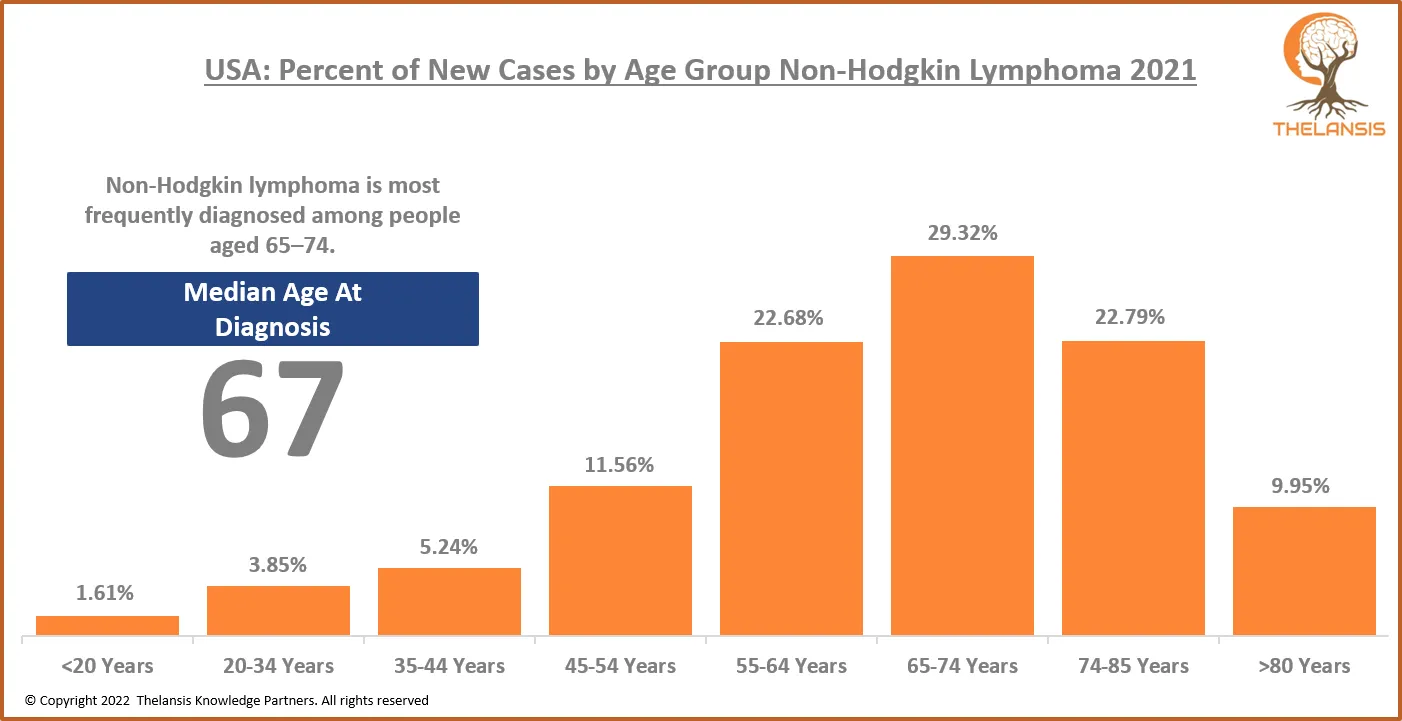
USA: Percent of New Cases by Age Group Non-Hodgkin Lymphoma 2021
[vc_row][vc_column][vc_custom_heading text="USA: Percent of New Cases by Age Group Non-Hodgkin Lymphoma 2021" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_r ...
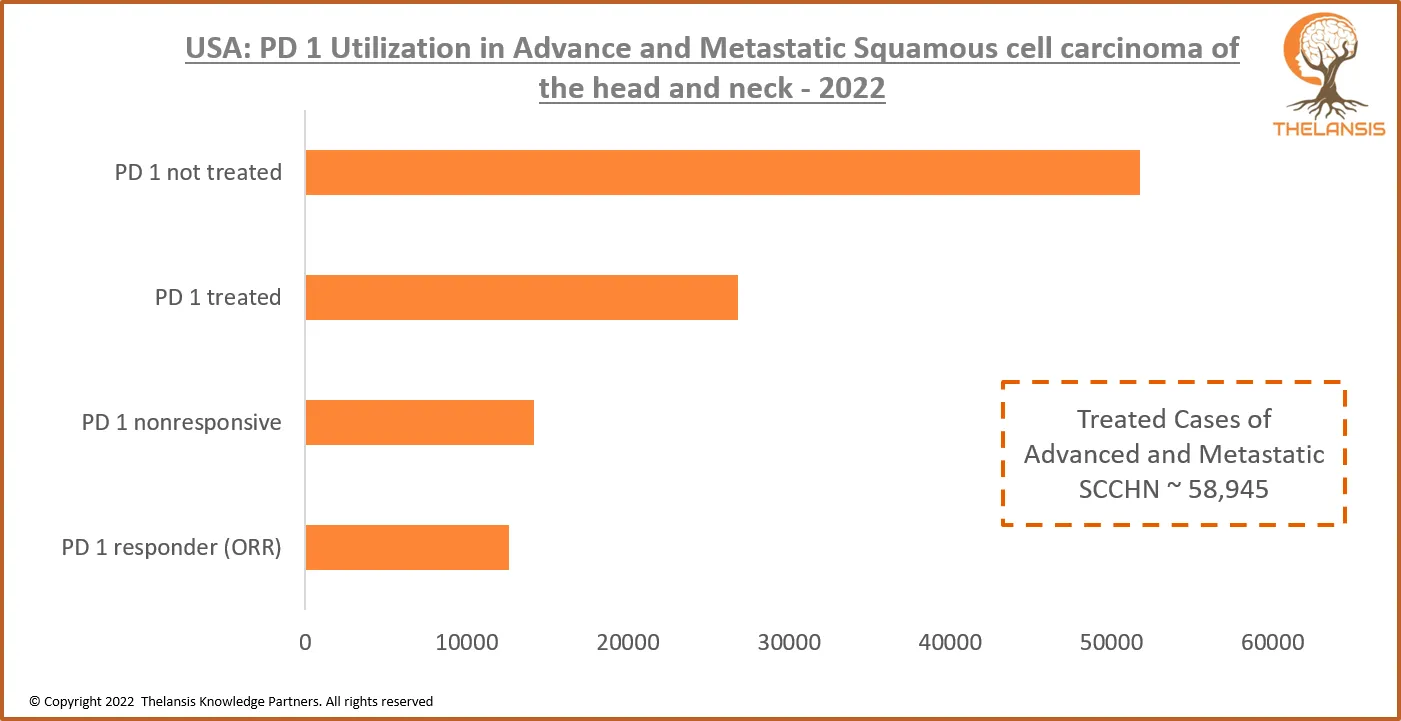
USA: PD 1 Utilization in Advance and Metastatic Squamous Cell Carcinoma of the Head and Neck – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Squamous Cell Carcinoma of the Head and Neck - 2022" font_container="tag:h2|text_align:center" use_theme_fon ...
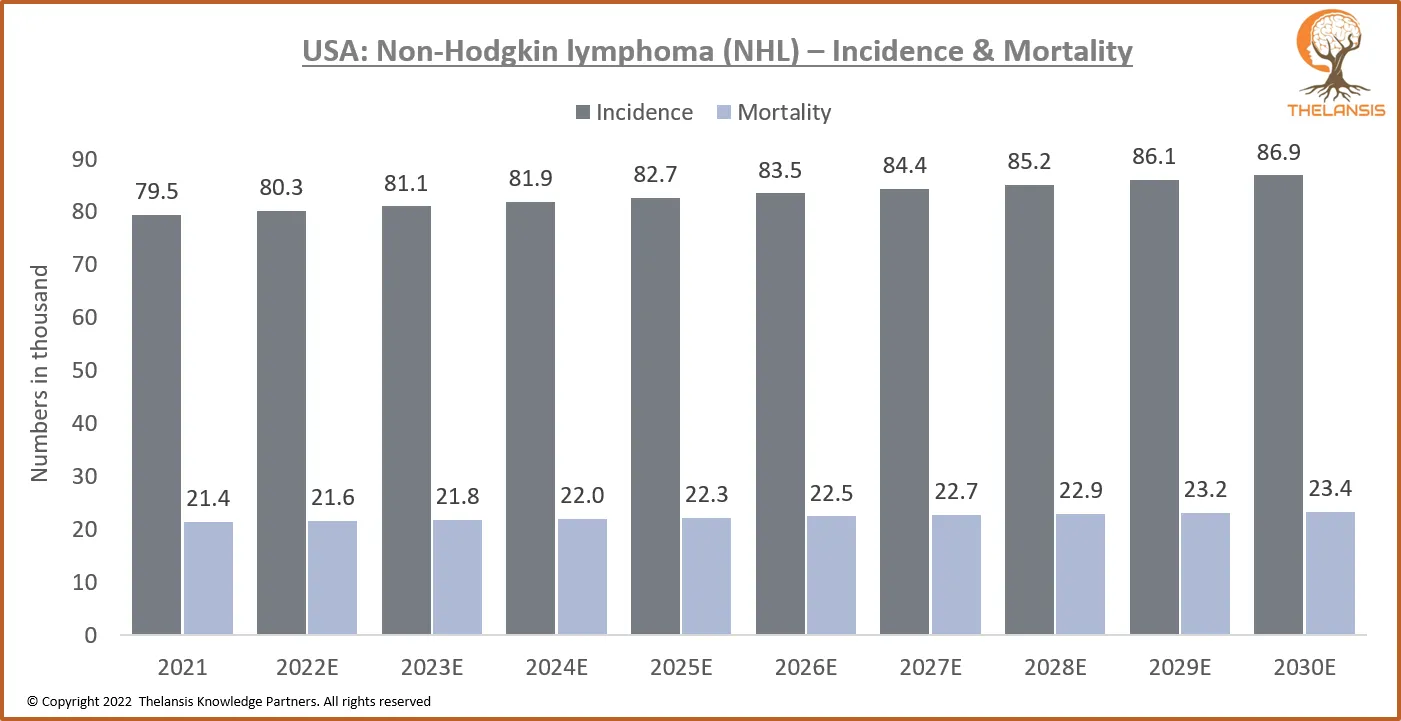
USA: Non-Hodgkin Lymphoma (NHL) – Incidence & Mortality
[vc_row][vc_column][vc_custom_heading text="USA: Non-Hodgkin Lymphoma (NHL) - Incidence & Mortality" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][v ...
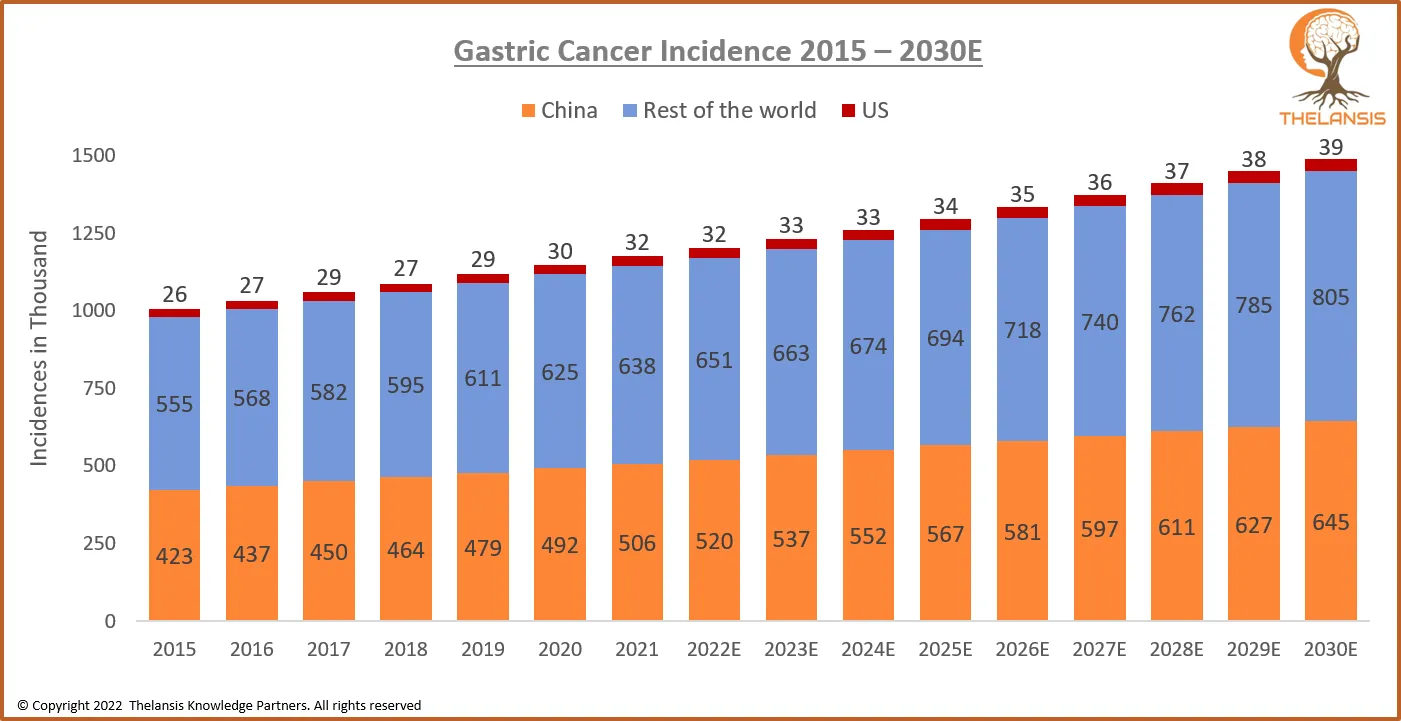
Gastric Cancer Incidence 2015 – 2030E
[vc_row][vc_column][vc_custom_heading text="Gastric Cancer Incidence 2015 – 2030E" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_cust ...

