Ikena Oncology’s IK-175 Receives FDA Fast Track Designation for Advanced Urothelial Carcinoma
Ikena Oncology, Inc. is a targeted oncology company that focuses on patient-directed cancer treatment has received Fast Track designation from the FDA for its drug IK-175, a novel aryl hydrocarbon rec ...
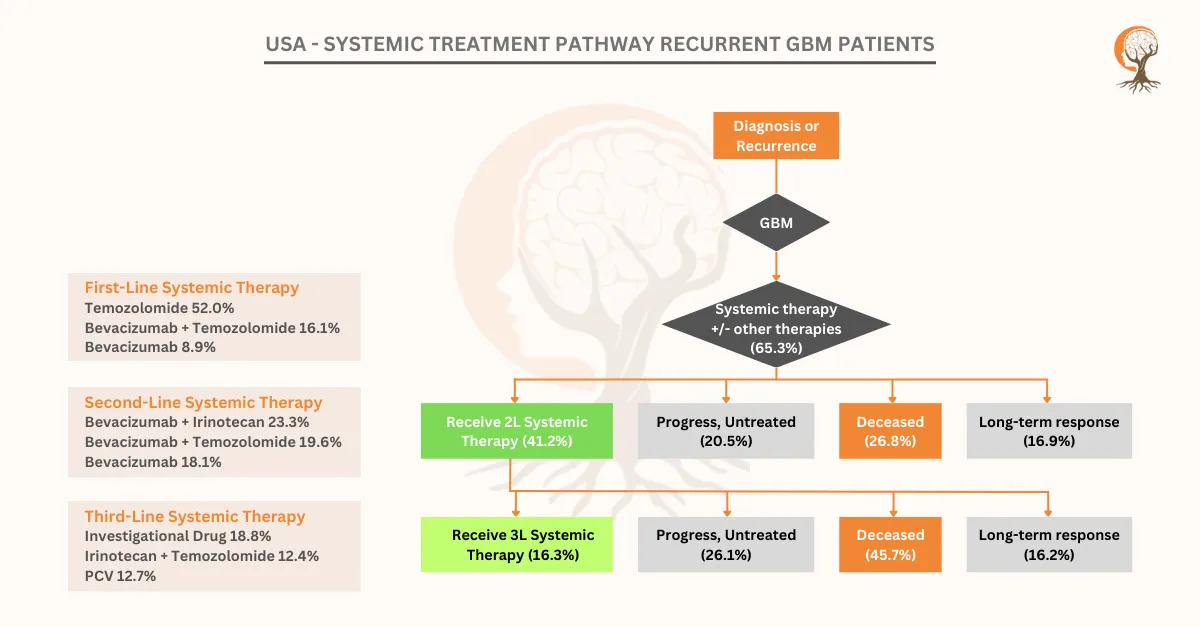
USA – Systemic Treatment Pathway Recurrent GBM Patients
[vc_row][vc_column][vc_custom_heading text="USA - Systemic Treatment Pathway Recurrent GBM Patients" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_co ...
FDA Approves Kevzara® (Sarilumab) for Treating Polymyalgia Rheumatica (PMR)
Regeneron Pharmaceuticals, Inc. and Sanofi have received FDA approval for Kevzara® (sarilumab) to treat polymyalgia rheumatica (PMR) in adult patients who have not responded well to corticosteroids or ...
SpringWorks Therapeutics’ Nirogacestat Granted Priority Review by FDA for Desmoid Tumor Treatment
SpringWorks Therapeutics, a clinical-stage biopharmaceutical company, has received acceptance from the U.S. Food and Drug Administration (FDA) for its New Drug Application (NDA) for nirogacestat, an i ...
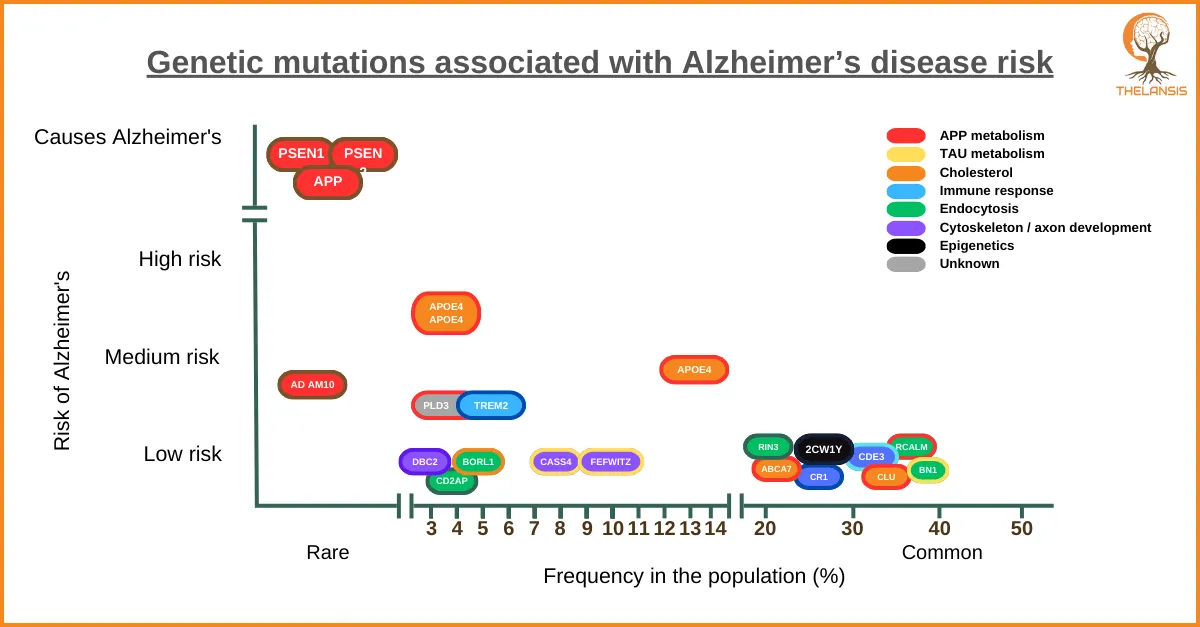
Genetic mutations associated with Alzheimer’s disease risk
[vc_row][vc_column][vc_custom_heading text="Genetic mutations associated with Alzheimer’s disease risk" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc ...

FDA Approves Apellis’ SYFOVRE™ (pegcetacoplan injection) for Geographic Atrophy (GA)
Apellis Pharmaceuticals, Inc. announced today that SYFOVRE™ (pegcetacoplan injection) has been approved by the United States Food and Drug Administration (FDA) for the treatment of geographic atrophy ...
PharmaDrug Inc. Announces Plans for First-In-Human Clinical Study with PD-001 in Esophageal Cancer
PharmaDrug Inc. plans to conduct a first-in-human study of its lead candidate, PD-001, an enteric-coated cepharanthine-2HCL, in Australia in H2 2023. PD-001 has the potential as a breakthrough therapy ...
Jubilant Therapeutics’ JBI-778 Granted Orphan Drug Designation by US FDA for Glioblastoma Multiforme Treatment
Jubilant Therapeutics, a clinical-stage biopharmaceutical company, has announced that JBI-778, an oral PRMT5 inhibitor, has received orphan drug designation from the U.S. Food and Drug Administration ...
IASO Bio’s CT103A Receives RMAT & Fast Track Designation from FDA for Treating Relapsed/Refractory Multiple Myeloma
IASO Biotherapeutics, a clinical-stage biopharmaceutical company, today announced that the United States Food and Drug Administration (FDA) has granted both Regenerative Medicine Advanced Therapy (RMA ...
Everest Medicines Announces South Korea Grants Fast-Track Review Designation to Nefecon for Primary IgA Nephropathy Treatment
Everest Medicines, a biopharmaceutical company developing and commercializing innovative pharmaceuticals and vaccines in Asia, has received Global Innovative product on Fast Track (GIFT) designation f ...

