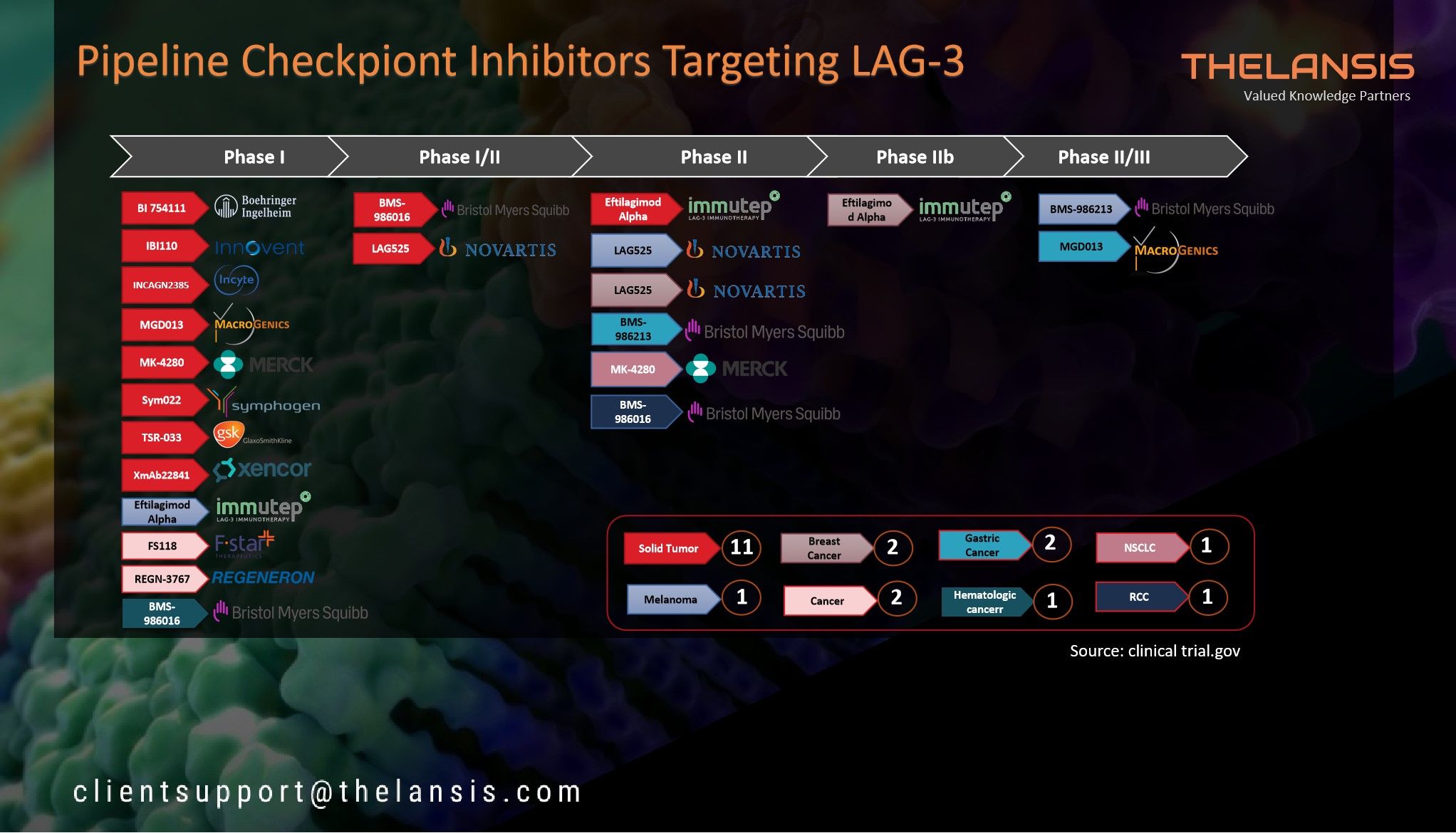
Crohn’s Disease (CD) – Press Release
According to Thelansis, the global Crohn’s disease (CD) market was estimated at ~$6,425 MN in 2020 and expected to reach ~$ 12,346 MN by the end of 2030 (10 years of markets forecasting). They attribu ...
Pulmonary Arterial Hypertension (PAH) – Press Release
Pulmonary Arterial Hypertension (PAH) – Market Outlook, Epidemiology, Market Forecast And Competitive Landscape Report – 2020 To 2030, provides the detailed therapy area landscape comprised of disease ...
FDA approves Apic Bio’s APB-102 for SOD1 Amyotrophic lateral sclerosis
Apic Bio, Inc. today announced that the US FDA had cleared its IND application for APB-102 to treat SOD1 Amyotrophic lateral sclerosis (ALS) – a common cause of familial ALS. APB-102 is a next-generat ...