BridgeBio Pharma announced FDA granted approval to Truseltiq for advanced Cholangiocarcinoma
The FDA granted accelerated approval to infigratinib for previously treated locally advanced or metastatic Cholangiocarcinoma harbors FGFR2 fusion or rearrangement. Infigratinib (Truseltiq; BridgeBio Pharma/QED Therapeutics, Helsinn Group) is an oral FGFR1-3 selective inhibitor in development for people with FGFR-driven conditions, including Cholangiocarcinoma, urothelial carcinoma, and achondroplasia.
Publish Date: 31-05-2021 Source: BridgeBio Pharma
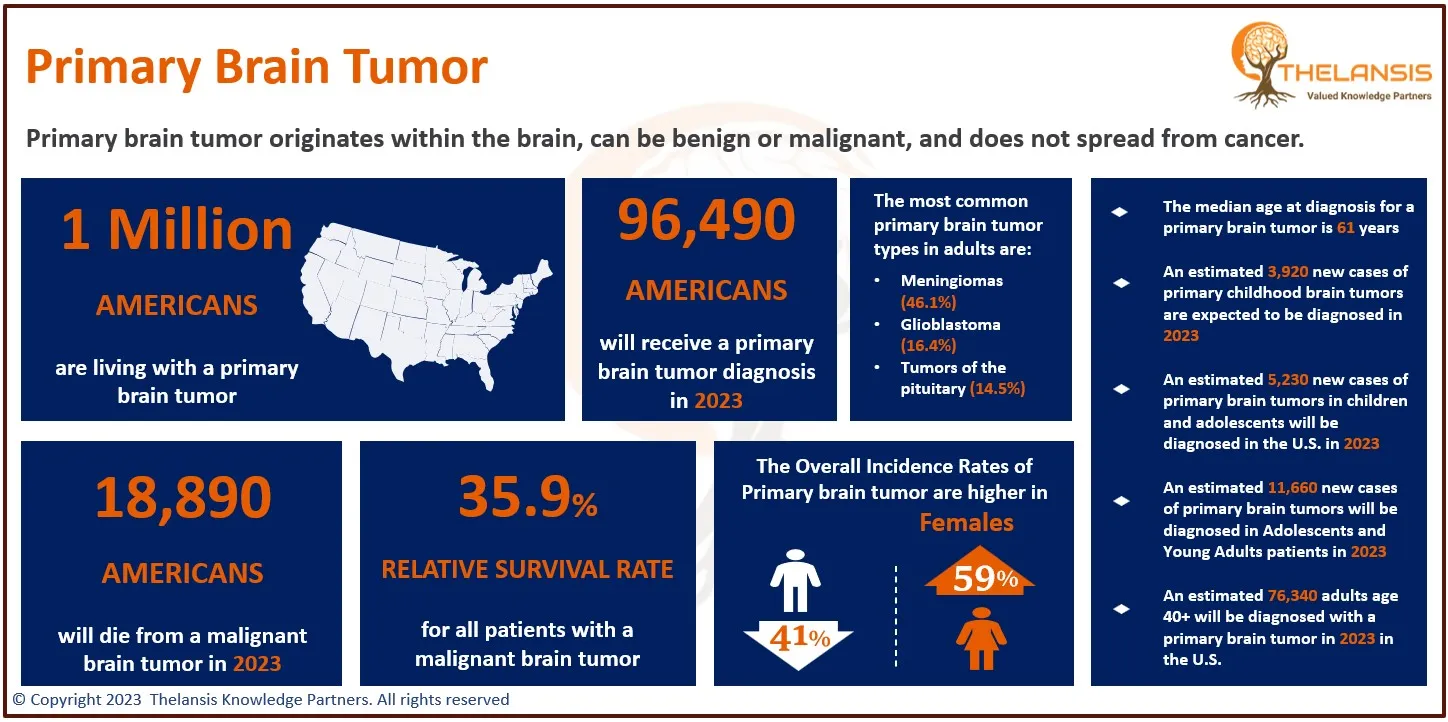
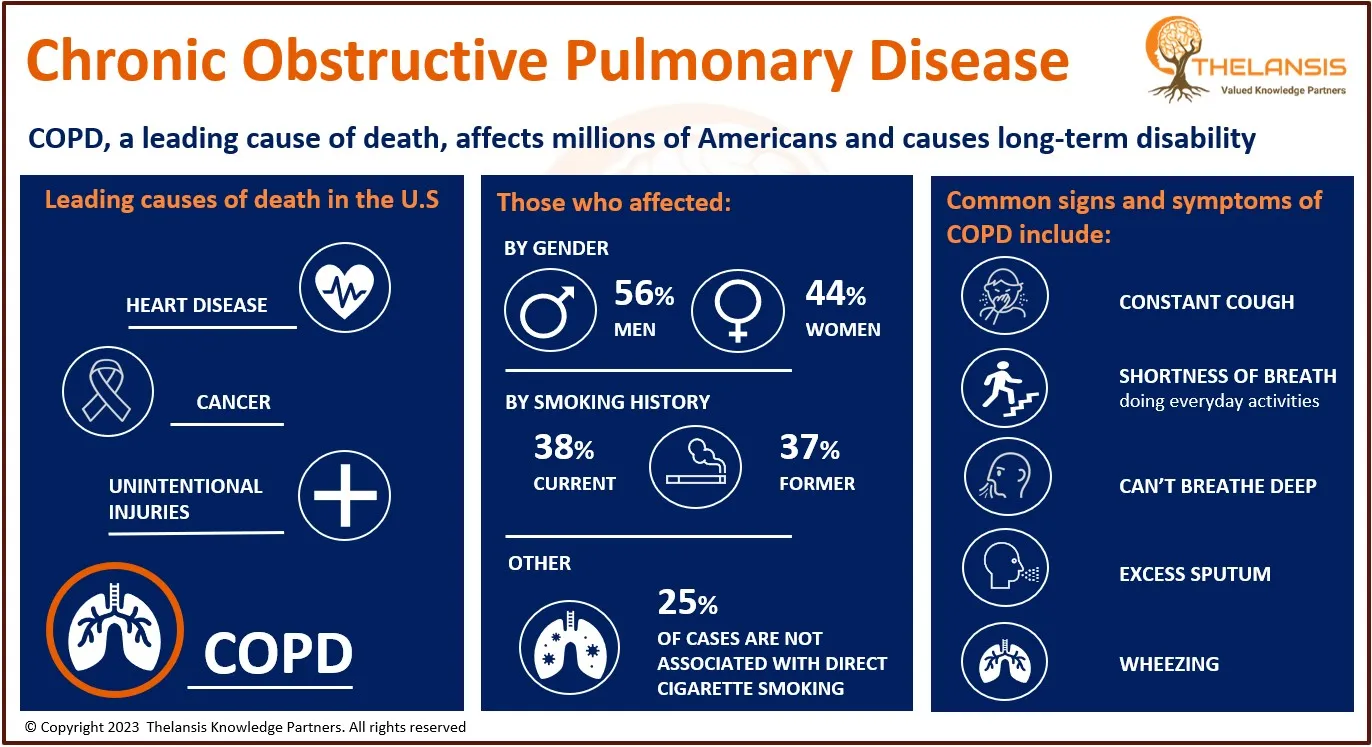
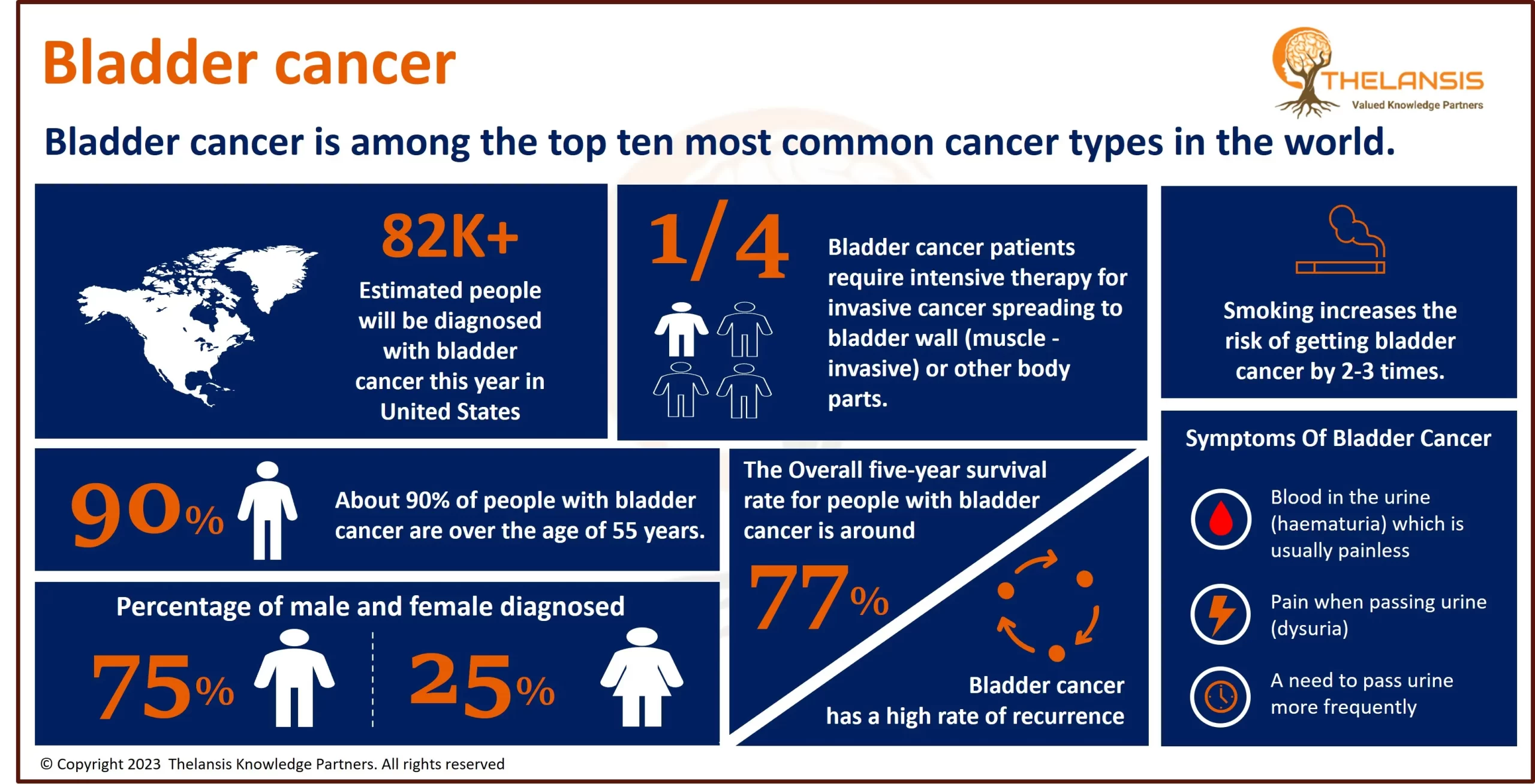
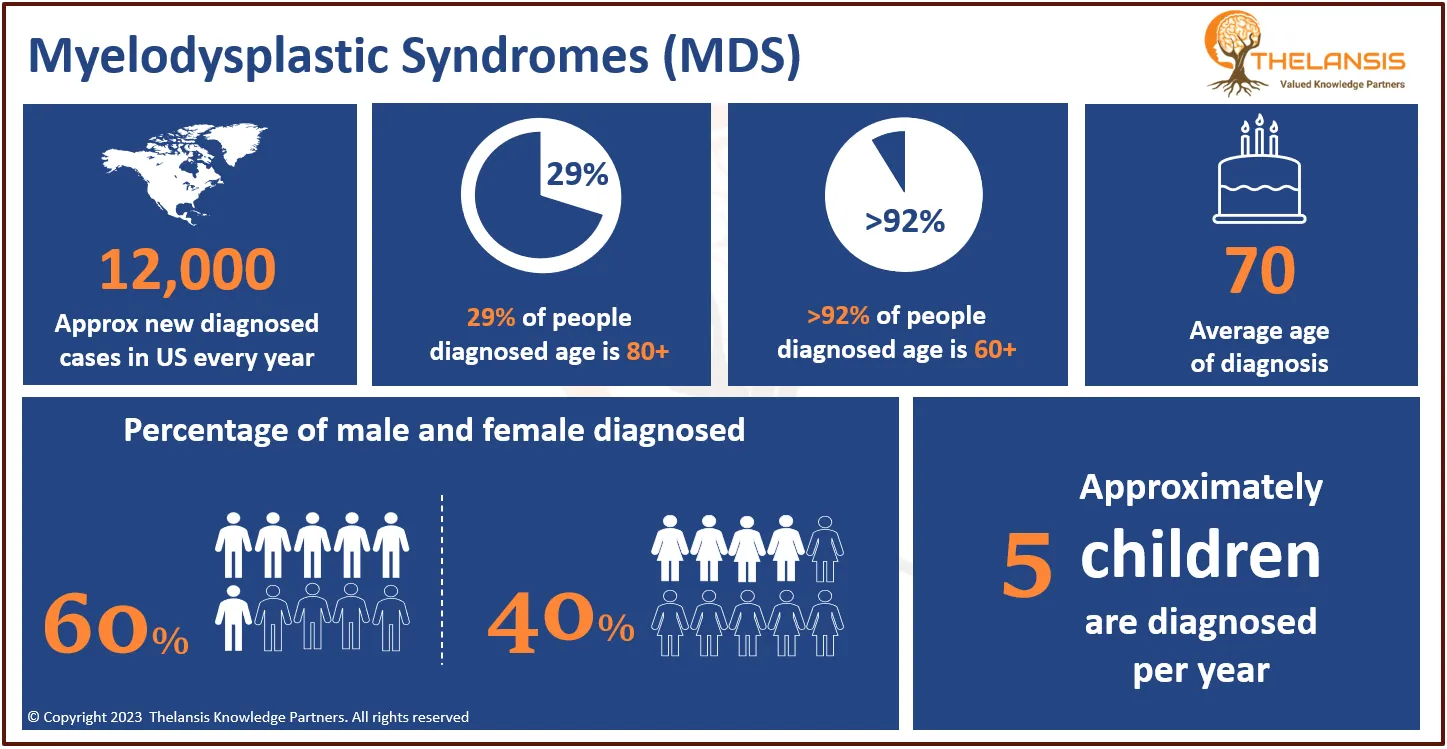
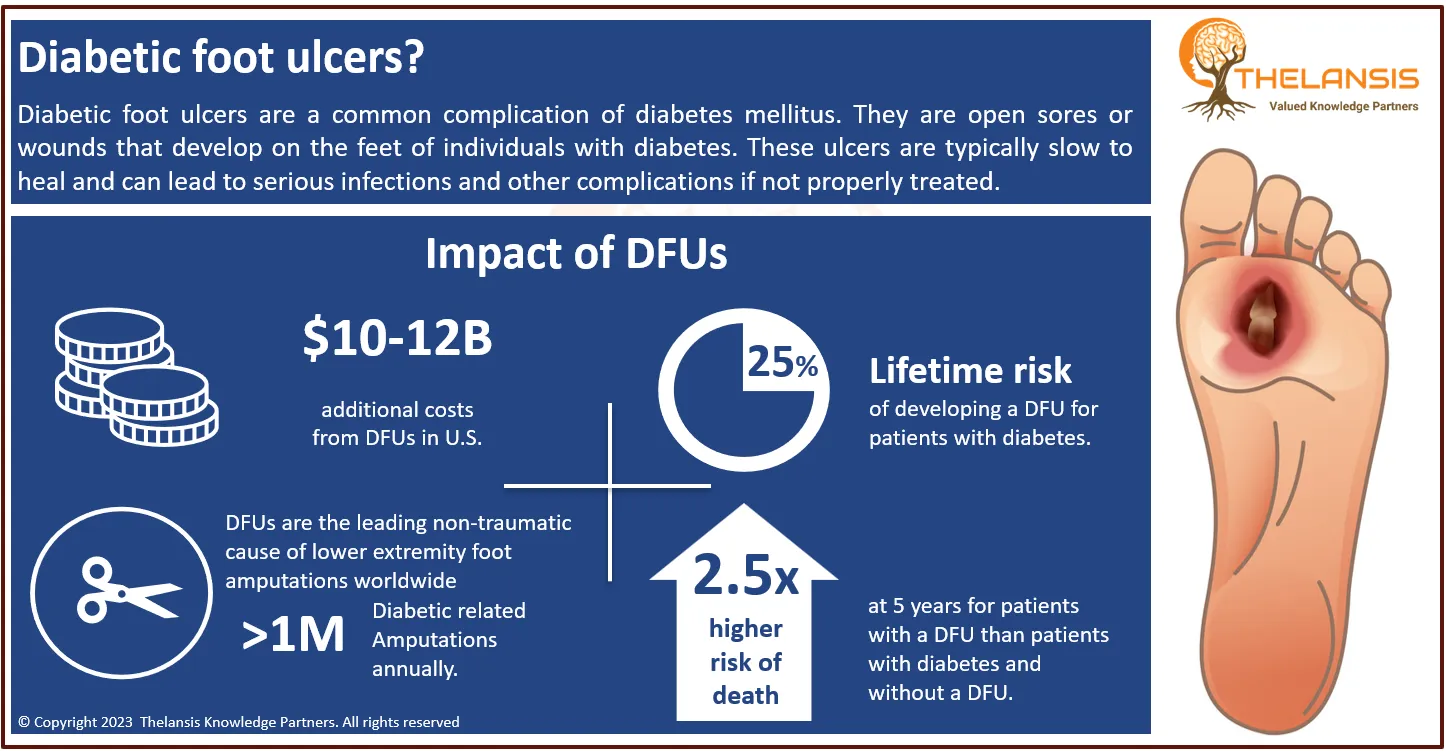
Cholangiocarcinoma is the 2nd most common primary liver tumor after hepatocellular carcinoma, accounting for about 3% of all gastrointestinal tumors. Most biliary tract cancers are adenocarcinomas with varying degrees of differentiation (mostly well-differentiated adenocarcinomas), though adenosquamous, squamous, mucinous, and anaplastic carcinomas are also seen. Cholangiocarcinoma can be classified anatomically based on tumor location within the biliary tree into intrahepatic, perihilar, and distal subtypes.
- In the US, approximately ~3,500 hospitalizations of Cholangiocarcinoma occur with an average incidence of between 0.45 to 2 cases per 100,000 population per year.
However, the current Cholangiocarcinoma treatment market share, market uptake, and attribute analysis for the most promising emerging therapies (Pemigatinib, AG-120 derazantinib, BGJ398, TAS-120, GNS561, Bintrafusp alfa, BBI503, urvalumab, Regorafenib, and others) have been provided in the study’s market outlook section, which covers eight MM countries: the United States, EU5 (Germany, Spain, France, Italy, UK), Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Cholangiocarcinoma treatment. The key companies in the advanced development stage are Incyte Corporation, Agios Pharmaceuticals, Basilea Pharmaceutical, QED Therapeutics, Taiho Oncology, Merck Serono, Sumitomo Dainippon Pharma, AstraZeneca, Bayer, etc., targeting Cholangiocarcinoma.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Cholangiocarcinoma to provide a clear understanding of the disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com