FDA approves Apic Bio's APB-102 for SOD1 Amyotrophic lateral sclerosis
Apic Bio, Inc. today announced that the US FDA had cleared its IND application for APB-102 to treat SOD1 Amyotrophic lateral sclerosis (ALS) – a common cause of familial ALS. APB-102 is a next-generation recombinant adeno-associated virus (AAV) capsid and micro ribonucleic acid (miRNA) vector constructs that have been shown to suppress the activity of the mutated SOD1 gene in preclinical proof-of-concept studies to slow or reverse the progression of SOD1 ALS.
Publish Date: 19-05-2021 Source: Apic Bio, Inc.
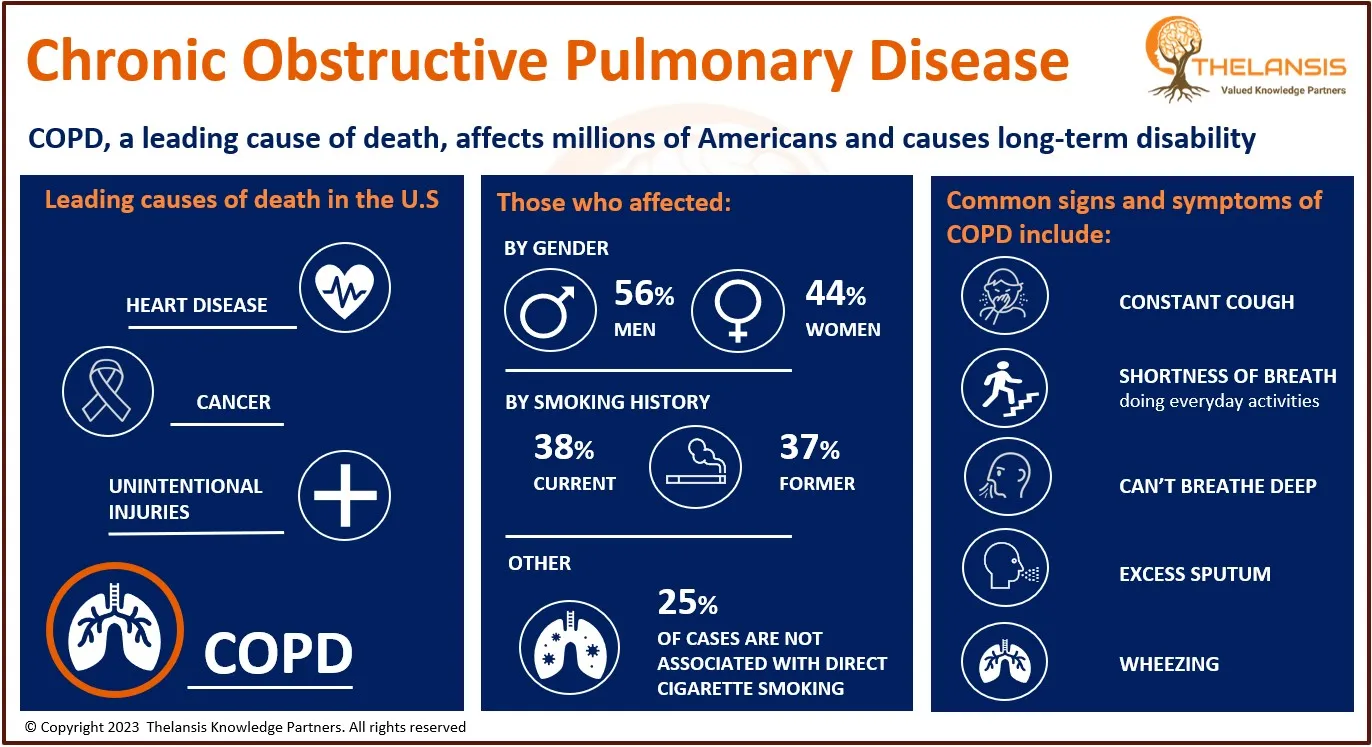
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that involves the progressive degeneration of the motor neurons of the cerebral cortex, brain stem, and spinal cord. Signs of UMN dysfunction include spasticity, weakness, slowness of movement, and brisk deep tendon reflexes. In contrast, LMN dysfunction signs include muscle fasciculation (spontaneous contraction affecting a small number of muscle fibers), wasting, and weakness.
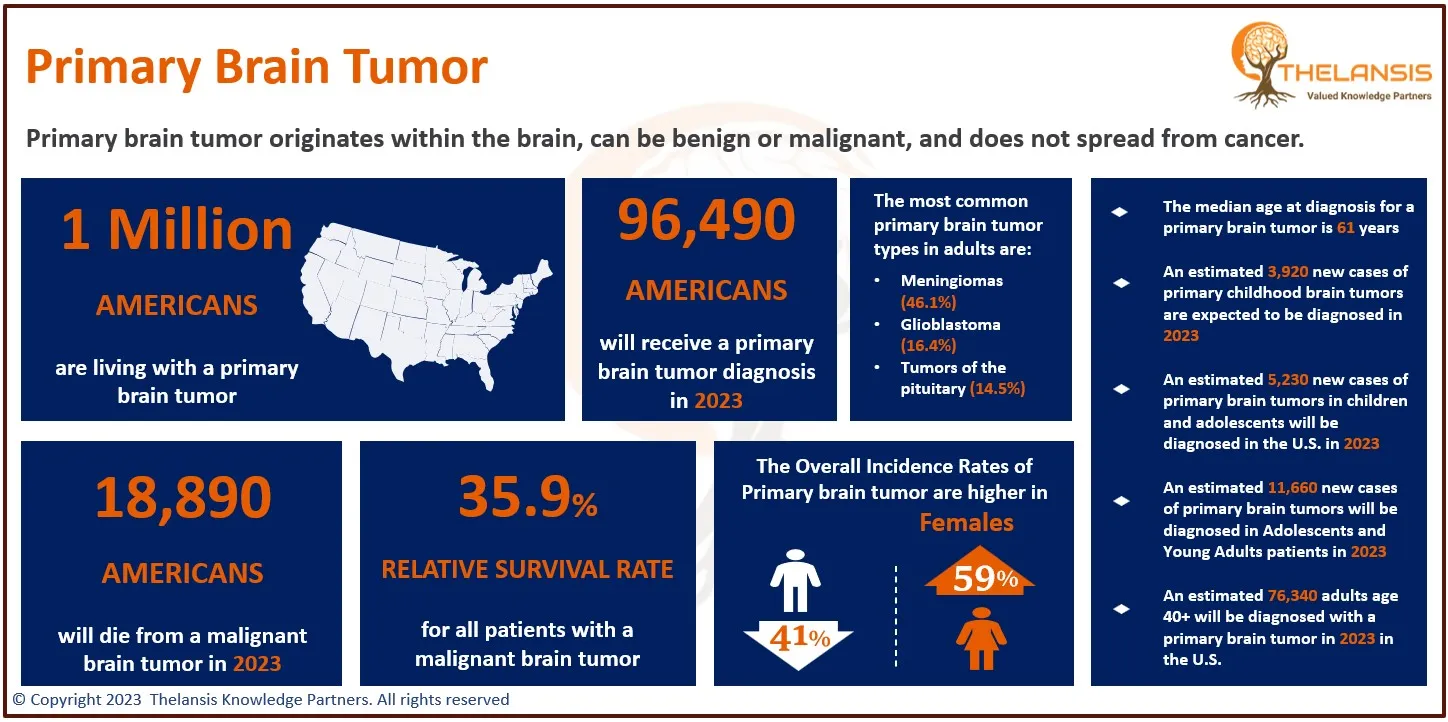
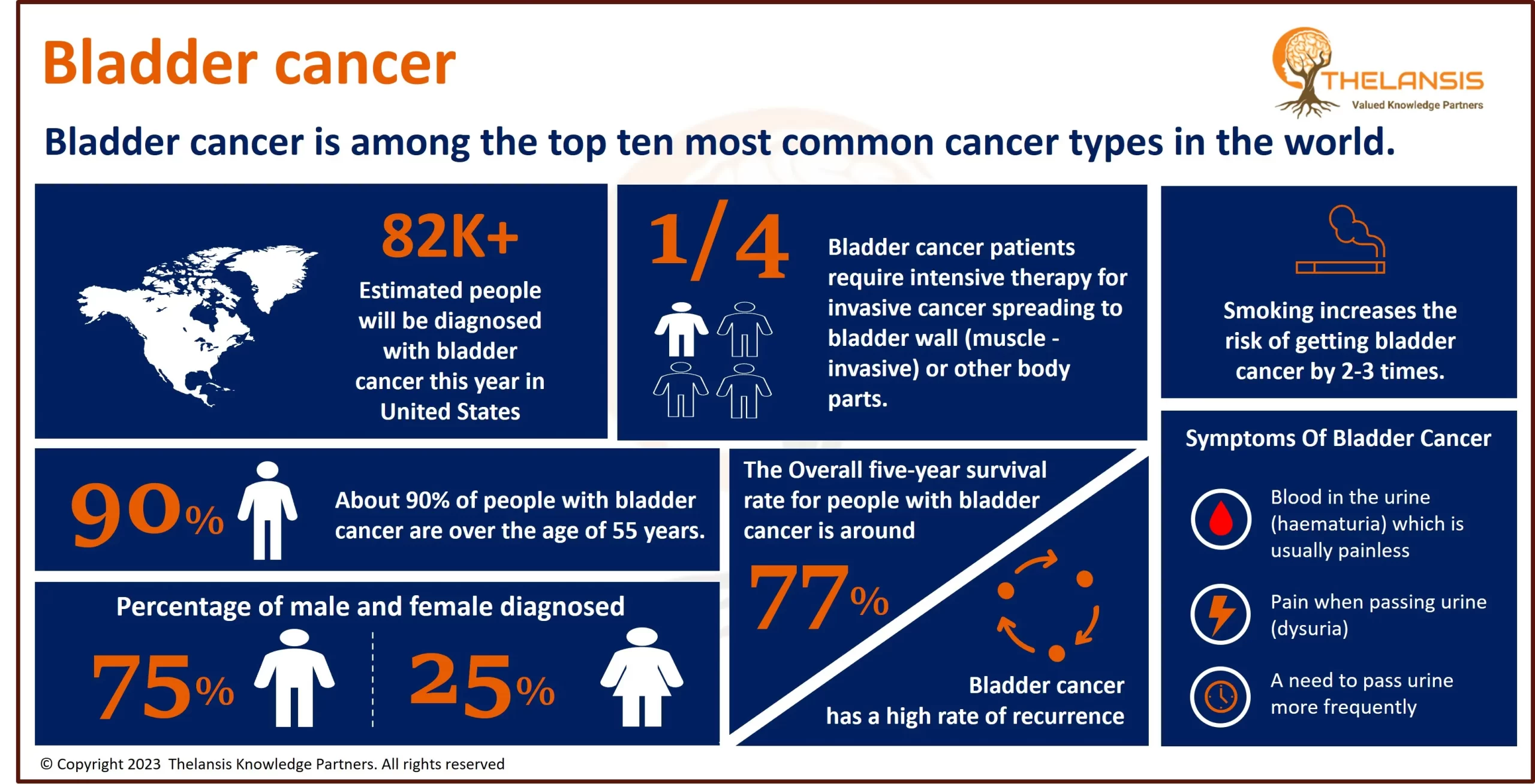
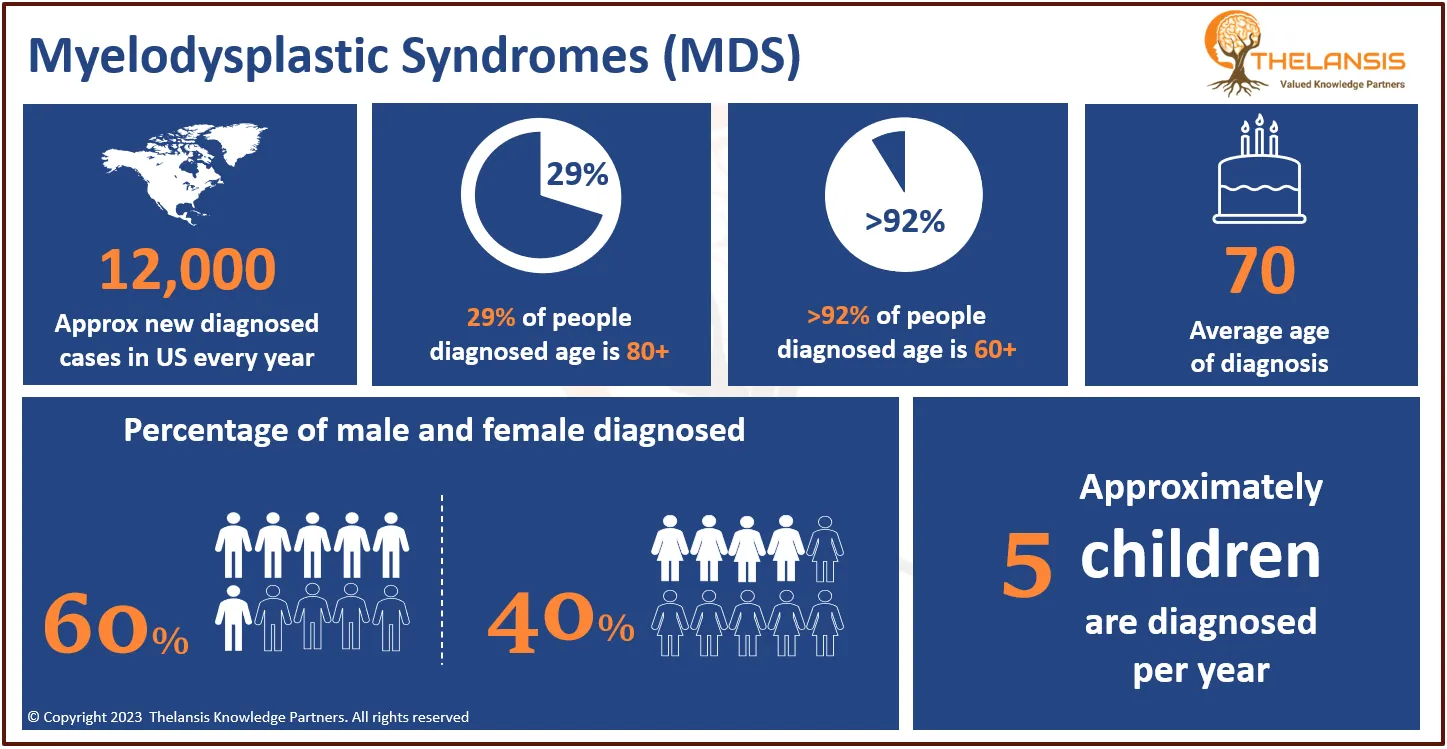
- Based on the epidemiology study of Thelansis, the incidence diagnosed cases of Approximately 5600 people in the United States are diagnosed with Amyotrophic lateral sclerosis each year. The annual incidence is 2-3 per 100,000 population; this is about equal to that of multiple sclerosis and five times higher than that of Huntington’s disease.
However, the current Amyotrophic lateral sclerosis treatment market share, market uptake, and attribute analysis for the most promising emerging therapies (Rilutek, Radicava, Tiglutik, Arimoclomol, IONIS-SOD1Rx, Masitinib, NurOwn, Simdax, TW001, TW001, AMX0035, EPI-589, GM604, NP001, NSI-566, Reldesemtiv, RNS60, RNS60, etc.) has provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for Amyotrophic lateral sclerosis treatment. The key companies in to advance stage of results are Orphazyme, Biogen, AB Science SA, BrainStorm Cell Therapeutics, Tenax Therapeutics, Treeway biotechnology, Grifols, Amylyx Pharmaceuticals, Dainippon Sumitomo Pharma, Genervon Biopharmaceuticals, Neuraltus Pharmaceuticals, etc. targeting ALS.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Amyotrophic lateral sclerosis (ALS) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com