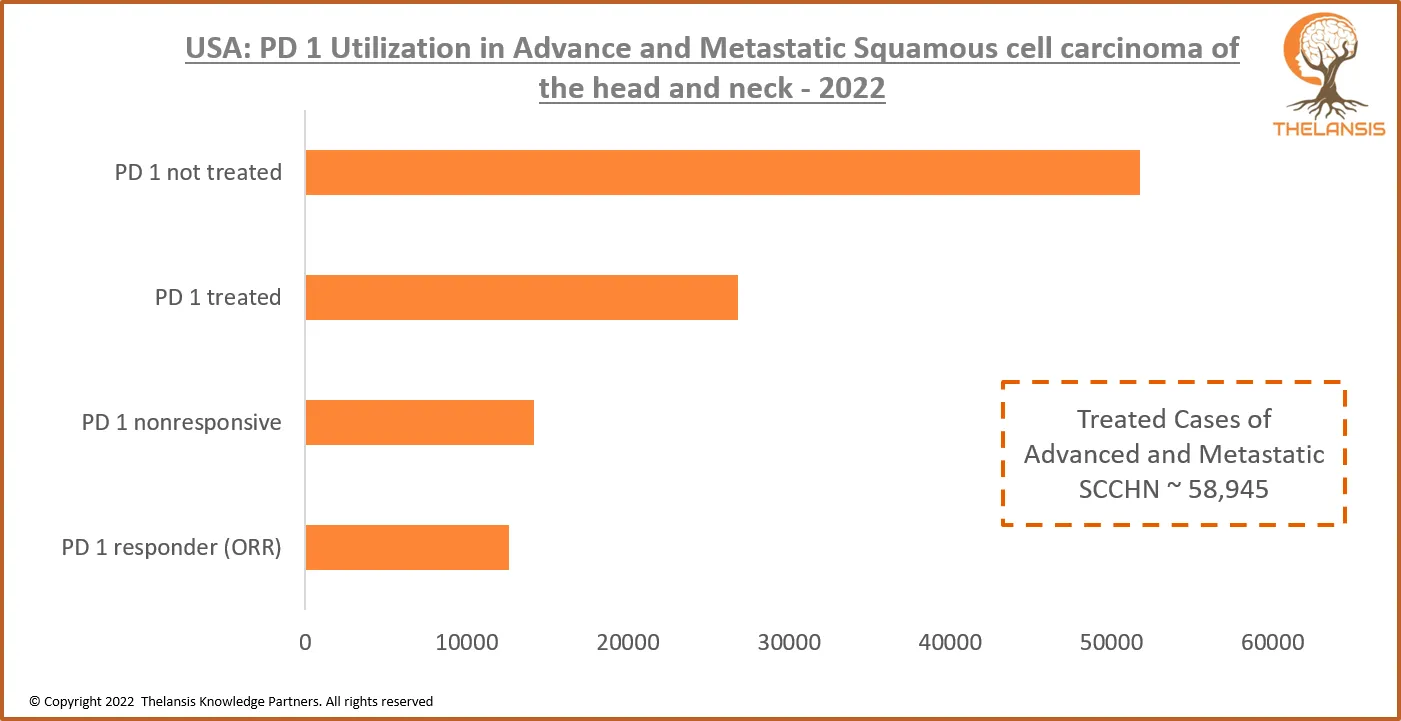
USA: PD 1 Utilization in Advance and Metastatic Squamous Cell Carcinoma of the Head and Neck – 2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic Squamous Cell Carcinoma of the Head and Neck - 2022" font_container="tag:h2|text_align:center" use_theme_fon ...

