Candel Therapeutics Receives U.S. FDA Fast Track Designation for CAN-2409 to Treat Stage III/IV NSCLC Patients
Candel Therapeutics receives FDA's fast track designation for CAN-2409, a viral immunotherapy developed to improve survival in combination with valacyclovir and pembrolizumab to treat stage III/IV non ...

Regenxbio’s RGX-202 Gene Therapy for Duchenne Muscular Dystrophy Receives Fast Track Designation from FDA
Regenxbio Inc. has received a fast track designation from the FDA for its innovative gene therapy, RGX-202, aimed at treating Duchenne muscular dystrophy. This therapy has been designed to deliver and ...
Verismo Therapeutics Receives Fast Track Designation from FDA for SynKIR-110, a Novel CAR T Therapy for Mesothelioma Treatment
Verismo Therapeutics, a clinical-stage company specializing in CAR T technology, has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for its innovative drug, SynKIR-11 ...
Caribou Biosciences’ CB-011 Granted Fast Track Designation by FDA for Relapsed or Refractory Multiple Myeloma
Caribou Biosciences Ltd., a top-notch clinical-stage biopharmaceutical company specializing in CRISPR genome-editing, has recently received Fast Track designation from the U.S. Food and Drug Administr ...
Renovion’s ARINA-1 Receives Fast Track Designation from FDA for Prevention of BOS Progression in Bilateral Lung Transplant Patients
Renovion, Inc. has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for ARINA-1, a medicine developed for the prevention of bronchiolitis obliterans syndrome (BOS) prog ...
FDA Grants Fast Track Designation to EpicentRx’s RRx-001 for Severe Oral Mucositis Prevention in Head & Neck Cancer Patients
EpicentRx, Inc. has announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to RRx-001, a novel compound with direct NLRP3 inhibitory and Nrf2 upregulatory effect ...
Prestige Biopharma’s PBP1510 Granted Fast Track Designation by FDA for Advanced Pancreatic Cancer Treatment
Prestige Biopharma, a leading biopharmaceutical company, has secured Fast Track designation from the U.S. Food and Drug Administration (FDA) for its innovative drug, PBP1510 (International Non-proprie ...
Everest Medicines Announces South Korea Grants Fast-Track Review Designation to Nefecon for Primary IgA Nephropathy Treatment
Everest Medicines, a biopharmaceutical company developing and commercializing innovative pharmaceuticals and vaccines in Asia, has received Global Innovative product on Fast Track (GIFT) designation f ...

FDA Grants Fast Track Designation for Reneo Pharma’s Mavodelpar (REN001) for LCHAD Deficiency Treatment
Reneo Pharmaceuticals, Inc., a clinical-stage pharmaceutical company focused on developing and commercialising therapies for patients with rare genetic mitochondrial diseases granted Fast Track design ...
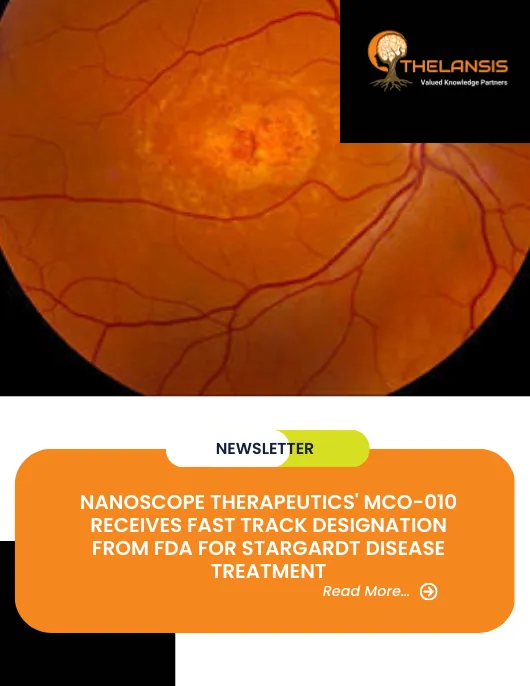
Nanoscope Therapeutics’ MCO-010 Receives Fast Track Designation from FDA for Stargardt Disease Treatment
Nanoscope Therapeutics, a clinical-stage biotech company specializing in gene therapies for retinal disorders, announced that the US FDA has granted Fast Track Designation to MCO-010. an ambient-light ...

