Jan 23 2023
/
IVERIC bio’s Avacincaptad Pegol Receives Breakthrough Therapy Status from FDA for Geographic Atrophy Treatment
IVERIC bio, Inc. announces U.S. FDA Breakthrough Therapy designation for avacincaptad pegol (Zimura®), a novel investigational complement C5 inhibitor for treating Geographic atrophy (GA) secondary to Age-Related Macular Degeneration (AMD). Based on 12-month primary endpoint data from the GATHER1 and GATHER2 trials, it’s the first therapy to receive Breakthrough Therapy designation status for this indication.
Publish Date: 23-01-2023 Source: IVERIC bio, Inc.
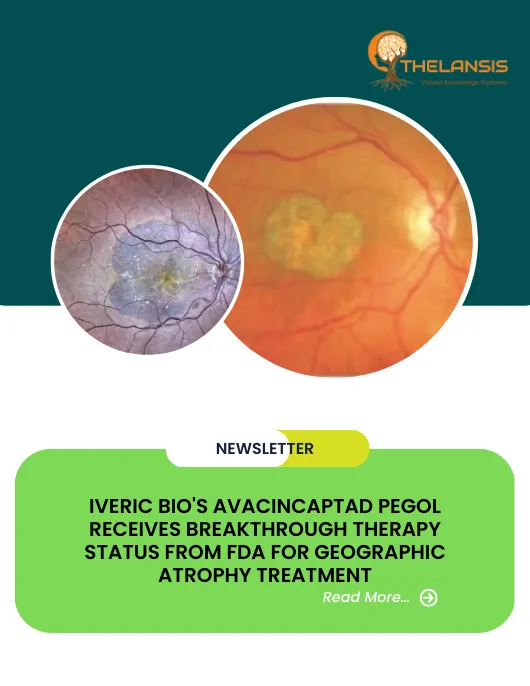
Geographic atrophy (GA) is defined as the late stage of the dry form of age-related macular degeneration (AMD). Some patients with age-related macular degeneration (AMD) may develop GA, which refers to regions of the retina where cells waste away and die (atrophy). The edge of GA was recognized as the termination of continuous RPE. Internal to the edge, there was an absence of RPE cells over wide areas, although a few RPE cells were scattered over the area of GA. The first visible alterations of AMD are drusen and retinal pigment epithelial (RPE) irregularities. This early stage of age-related maculopathy (ARM) may progress to either geographic atrophy or exudative AMD. Approximately 11% of patients with atrophic AMD eventually progress to the exudative form at four years of evolution. In some eyes, choroidal neovascularisation (CNV) also leads to GA.
- In the USA, the prevalence of GA is 3.5% in subjects older than 65 years, accounting for approximately half the prevalence of exudative AMD.
However, the current Geographic Atrophy (GA) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (ALK-001, Danicopan, etc.) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Geographic Atrophy treatment. The key companies in the advanced development stage are Alkeus Pharmaceuticals, Inc., Alexion Pharmaceuticals, etc., targeting GA.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Geographic Atrophy to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com - Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com
Related posts:
- Abbisko Therapeutics’ Pimicotinib (ABSK021) Receives Breakthrough Therapy Designation from FDA for Treatment of Tenosynovial Giant Cell Tumor
- Moderna’s mRNA-1345 Receives Breakthrough Designation from FDA for Respiratory Syncytial Virus (RSV) Prevention
- SAB Biotherapeutics’ SAB-176 Granted Breakthrough Therapy Designation by FDA for High-Risk Patients with Type A and B Influenza
- INOVIO’s INO-3107 Granted FDA Breakthrough Therapy Designation for RRP Treatment

