Jan 30 2023
/
Abbisko Therapeutics’ Pimicotinib (ABSK021) Receives Breakthrough Therapy Designation from FDA for Treatment of Tenosynovial Giant Cell Tumor
Abbisko Therapeutics today announced that its CSF-1R inhibitor Pimicotinib(ABSK021)has been granted the breakthrough therapy designation from FDA for the treatment of tenosynovial giant cell tumor (TGCT) patients that are not amenable to surgery. This breakthrough therapy designation approval is based on results from the phase Ib clinical trial of TGCT cohort for Pimicotinib.
Publish Date: 30-01-2023 Source: Abbisko Therapeutics Co., Ltd.
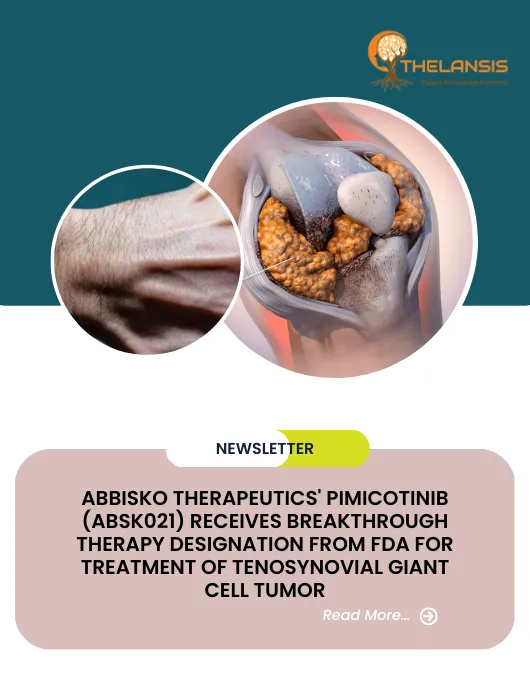
Tenosynovial giant cell tumour (TGCT) is a proliferative monoarticular lesion that occurs in and around joints throughout the body. TGCT is a group of lesions that develop from the synovium of joints, bursae, and tendon sheaths. There are two main subtypes of TGCT: localized-type TGCT (L-TGCT) and diffuse-type TGCT (D-TGCT). L-TGCT and D-TGCT behave differently and are categorized as separate clinical entities. Pain, swelling, stiffness, and limited range of motion are common symptoms. Other functional signs include instability, giving way and joint blockage. However, these nonspecific presentations can cause a delay in diagnosis and multiple visits to medical professionals. TGCT gene expression is consistent with apoptosis resistance, inflammation, and matrix degradation, resulting in ongoing proliferation and joint destruction. CD53, ALOX5AP, SPP1, and MMPs 1 and 9 are among the genes that are highly overexpressed. Complete excision of TGCT is the primary choice of treatment. Depending on the location, L-TGCT is often removed relatively easily via arthroscopy or open surgery, with a recurrence rate of around 10%.
- The incidence of localized-type TGCT (L-TGCT) ranges from 30 to 40 cases per million population, whereas L-TGCT and D-TGCT incidence cases range from 5 to 8 cases per million population.
However, the current Tenosynovial Giant Cell Tumor (TGCT) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (vimseltinib, Emactuzumab, FPA008, etc.) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Tenosynovial Giant Cell Tumor (TGCT) treatment. The key companies in the advanced development stage are Deciphera Pharmaceuticals LLC, SynOx Therapeutics Limited, Five Prime Therapeutics, Inc., etc., targeting TGCT.
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Tenosynovial Giant Cell Tumor (TGCT) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com - Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com
Related posts:
- Moderna’s mRNA-1345 Receives Breakthrough Designation from FDA for Respiratory Syncytial Virus (RSV) Prevention
- Abbisko Cayman’s ABSK012 Granted FDA Orphan Drug Designation for Soft Tissue Sarcoma Treatment
- SAB Biotherapeutics’ SAB-176 Granted Breakthrough Therapy Designation by FDA for High-Risk Patients with Type A and B Influenza
- INOVIO’s INO-3107 Granted FDA Breakthrough Therapy Designation for RRP Treatment

