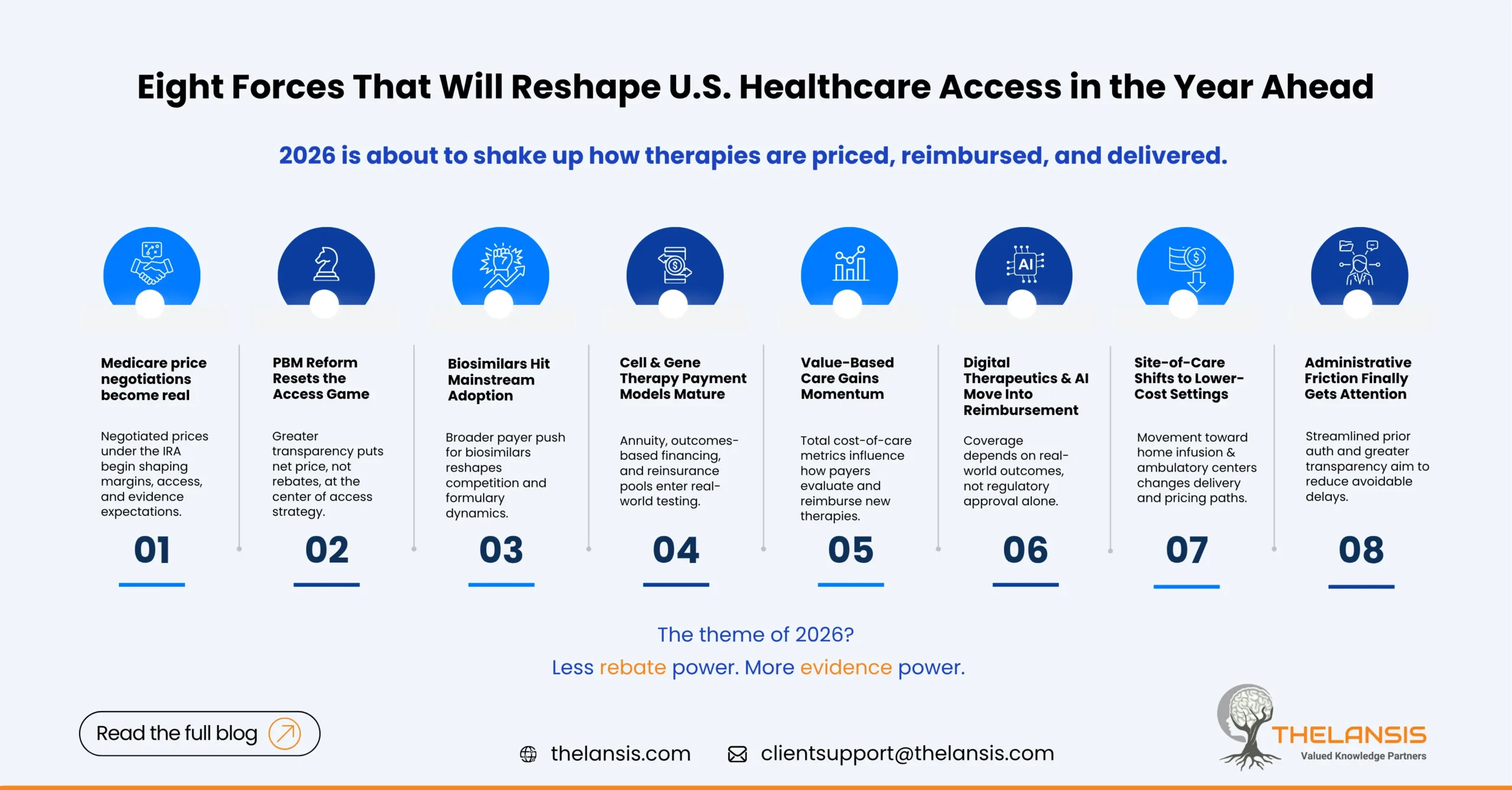
Eight Emerging U.S. Medical Cost & Access Drivers to Watch in 2026
The U.S. healthcare system never really sits still but 2026 is shaping up to be a year where cost, access, and innovation intersect in new and sometimes uncomfortable ways. From the Inflation Reductio ...
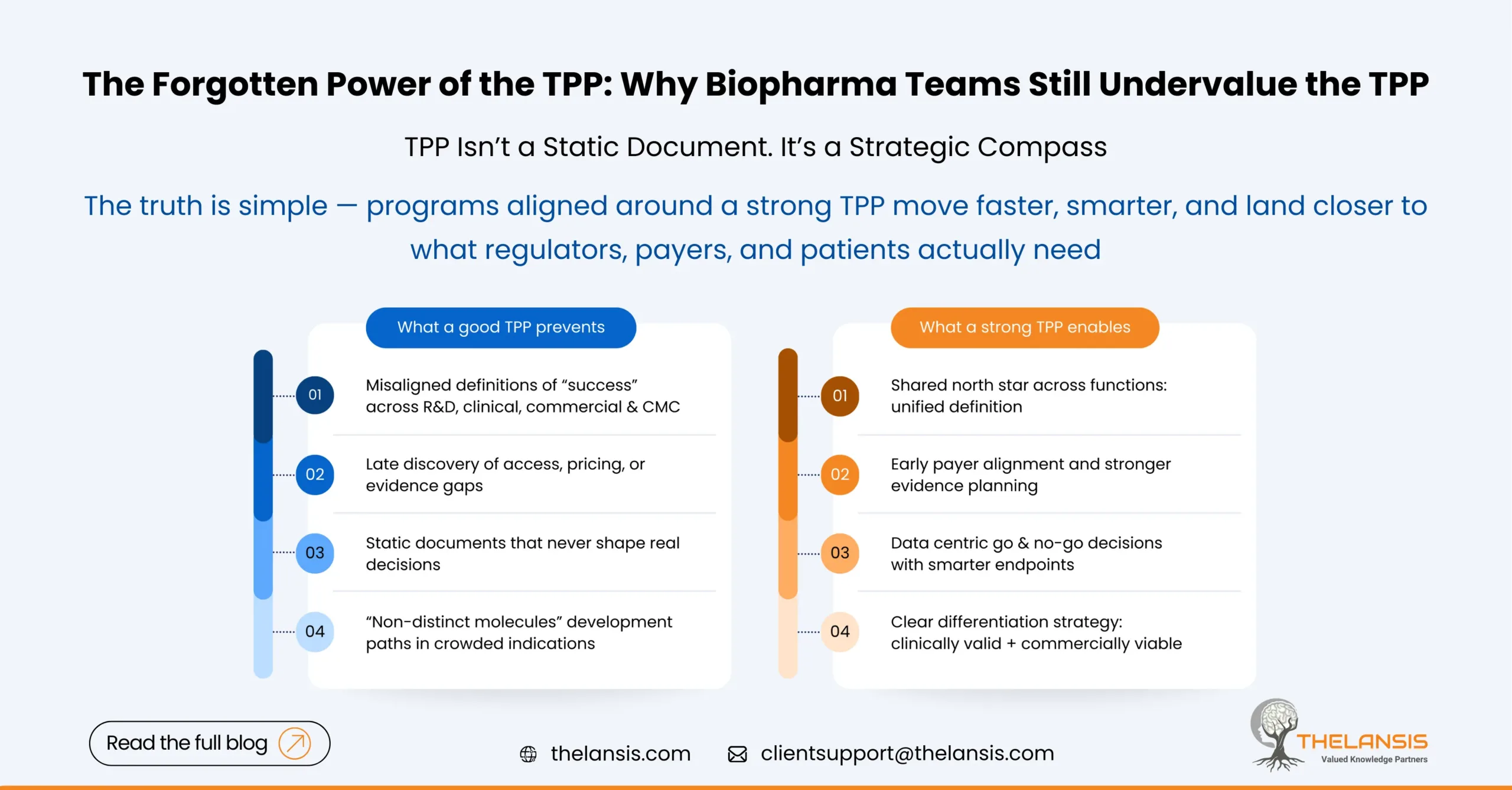
The Forgotten Power of the TPP: Why Many Biopharma Projects Fail Without It
In the fast‐moving world of biopharma innovation, it’s easy to get excited by the science – the novel target, the breakthrough molecule, the cutting-edge modality. But amid that excitement, one strate ...
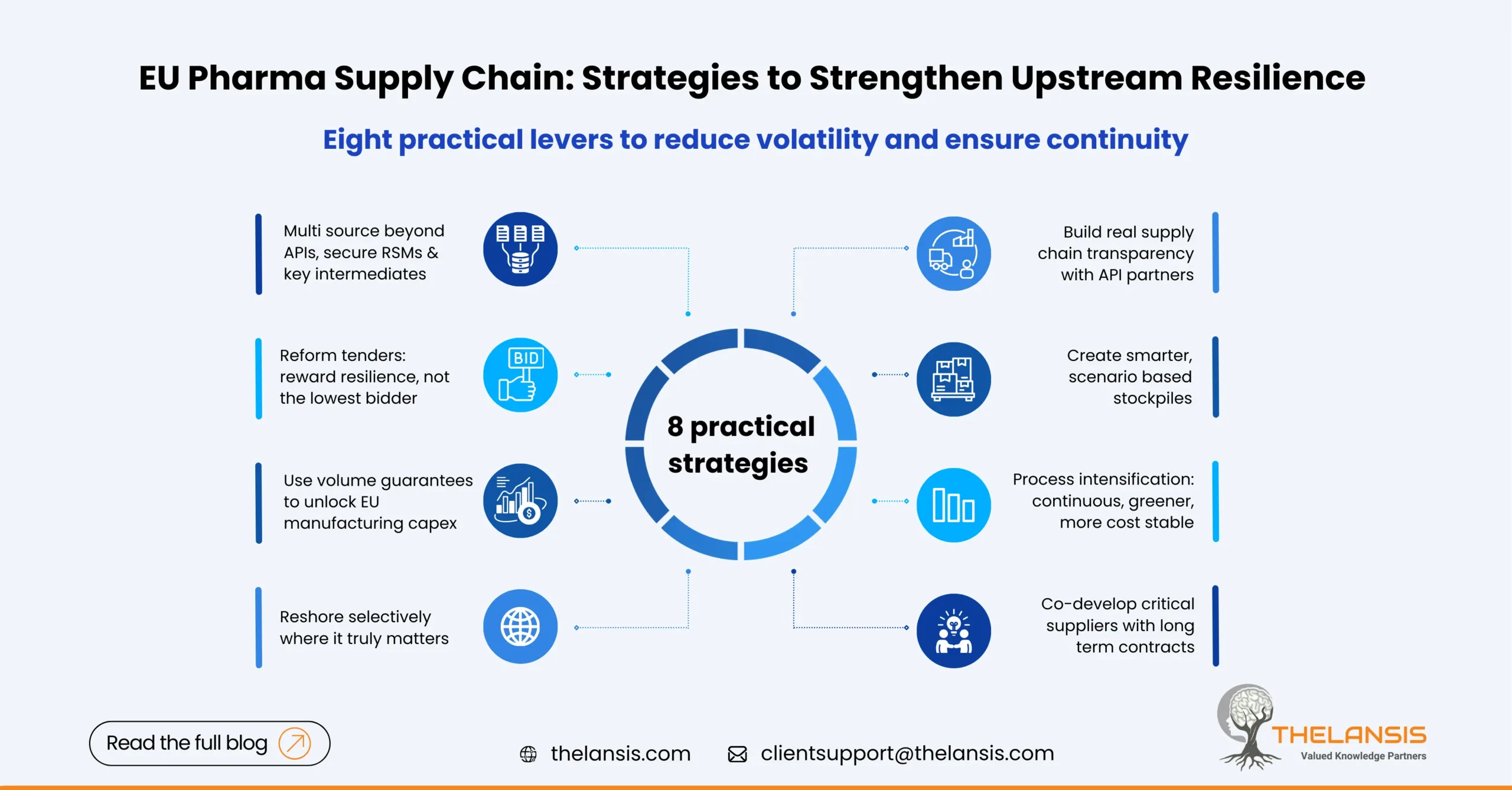
EU Pharmaceutical Supply Chain: How to Shrink Upstream Risk Without Shrinking Access
Europe’s pharmaceutical landscape is undergoing a significant change due to its medicine shortage problem, which has a stubborn root cause: fragile upstream links. The situation becomes apparent at th ...
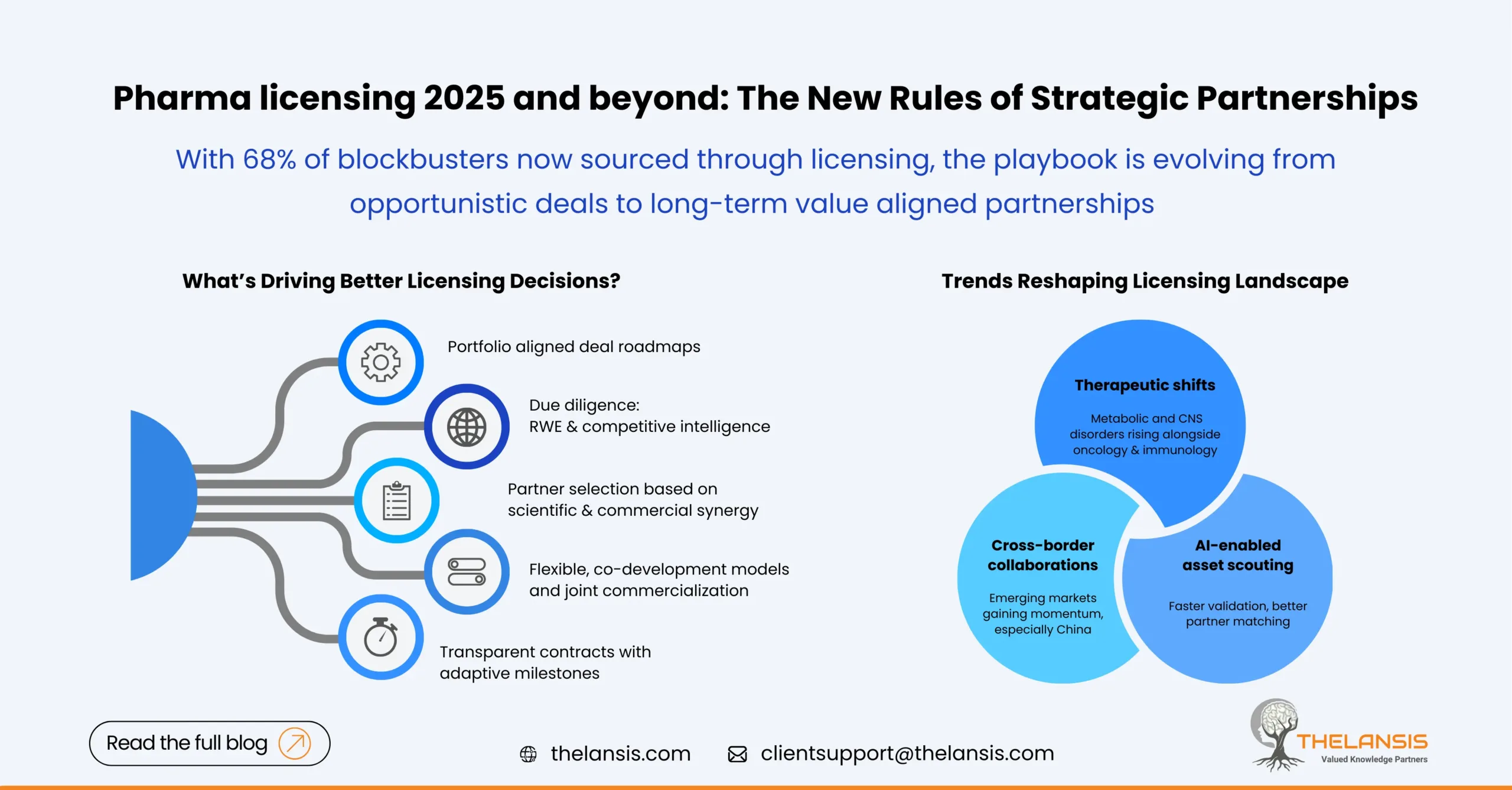
Pharma Licensing 2025 and Beyond: Rethinking Strategic Partnerships for Long-Term Value
Introduction: Licensing as a Growth Engine in Pharma The global pharmaceutical and biotech companies are entering an era of collaboration, agility, and strategic alliances. As the R&D cost rises ...
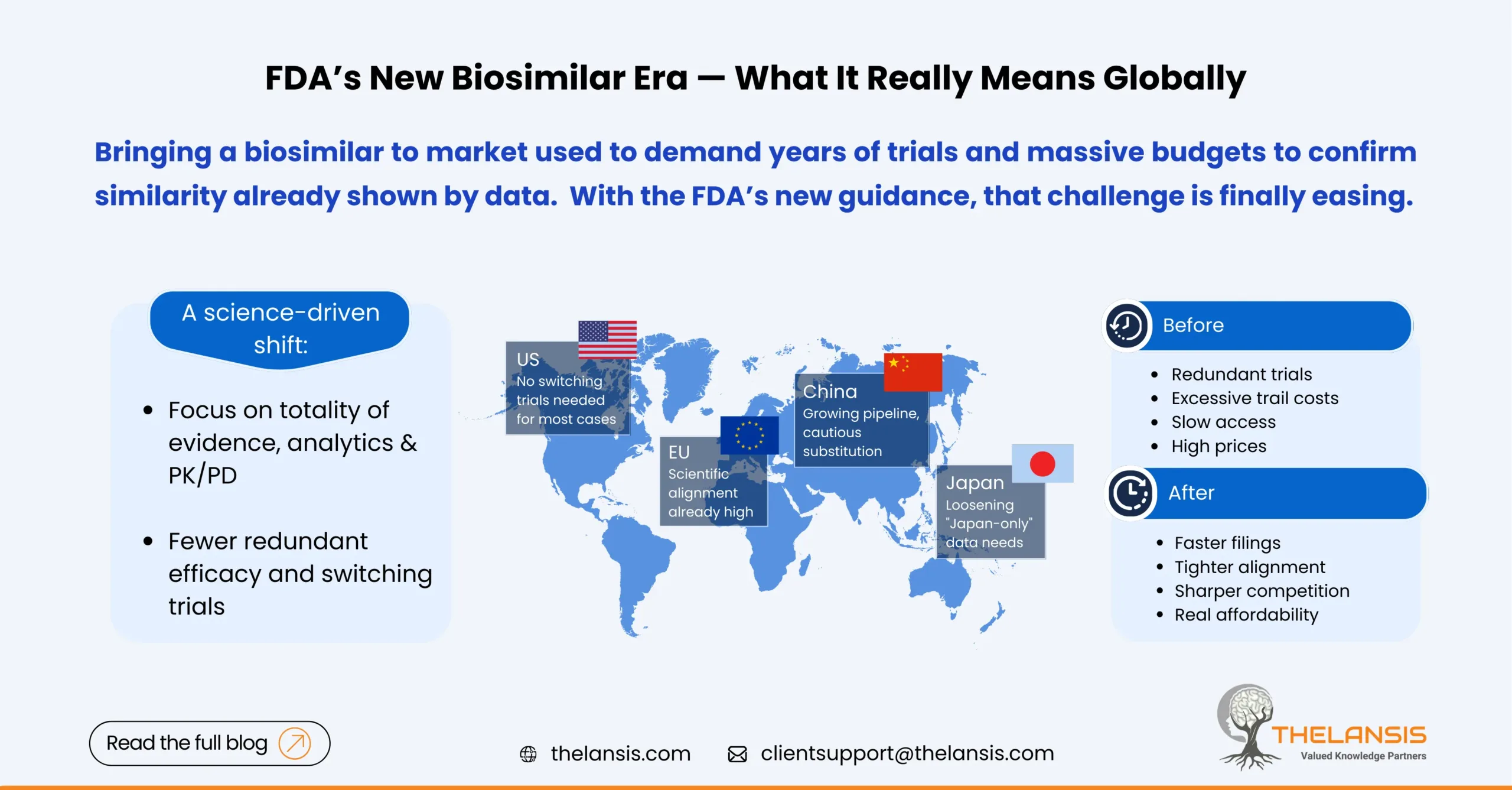
FDA’s Updated Biosimilar Policy: What it really means in the US, EU5, Japan, and China
The regulatory landscape for biosimilars is shifting significantly in 2025. The FDA has taken two big steps that collectively make it faster and cheaper to bring many biosimilars to market:In June 202 ...
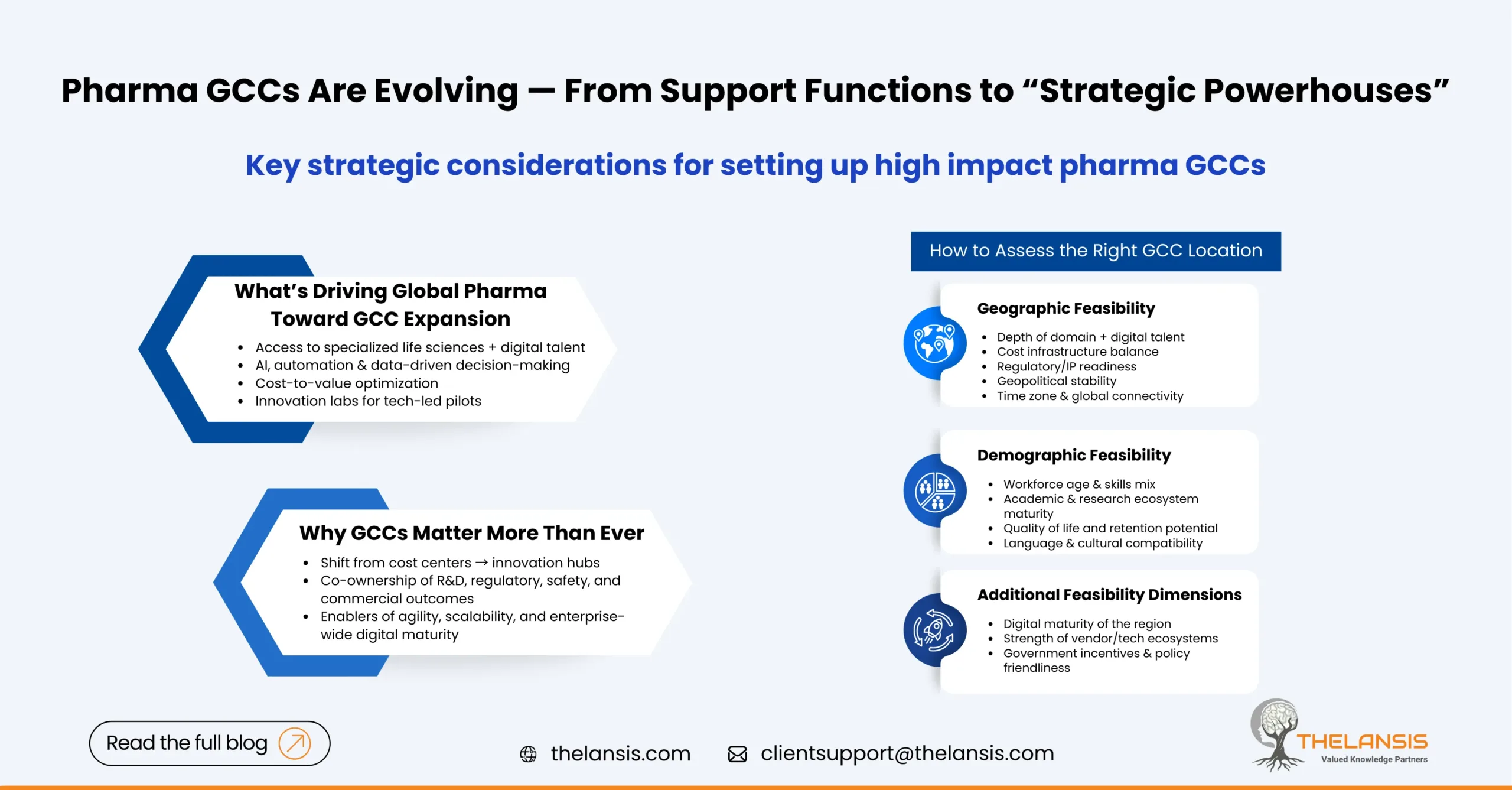
Pharma GCC Setup: A Strategic Feasibility Framework for Location and Talent Decisions
The pharma industry has seen a wave of change over the decades, driven by digital innovation, data analytics & global collaboration. A major catalyst behind this change has been the rise of Global ...

