
Why Most RWE Fails Payer Scrutiny (and What Actually Passes)
Real-world evidence is no longer optional in market access discussions. Payers expect it. HTA bodies ask for it. Internal teams invest heavily in generating it. And yet, a quiet reality persists: most ...
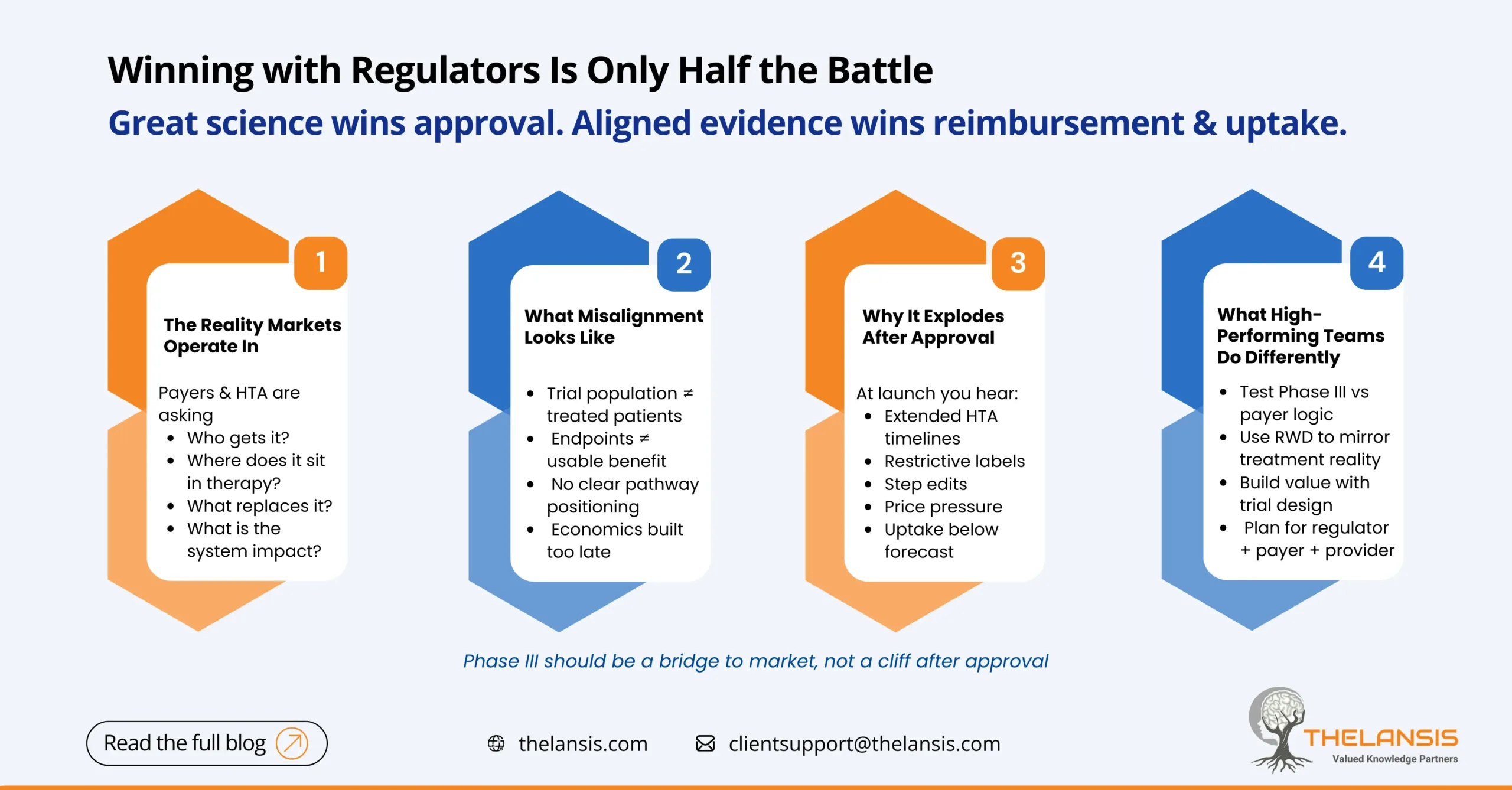
The Hidden Gap Between Phase III Success and Market Access: When Evidence Misalignment Delays Commercial Readiness
For many biopharma teams, Phase III is treated as the final mountain to climb. Once efficacy and safety are proven, commercial readiness is assumed to follow naturally. In reality, that assumption is ...
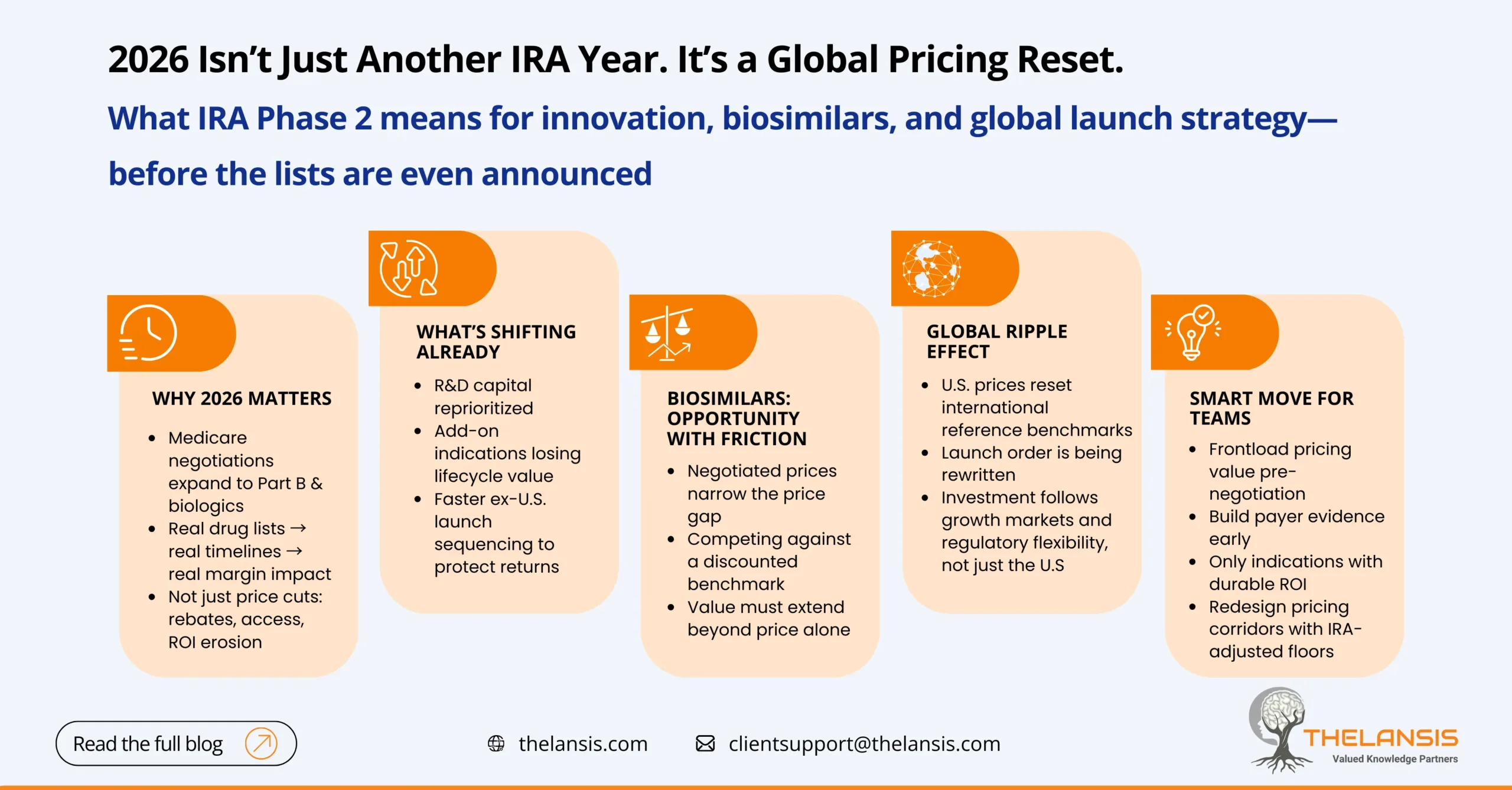
The IRA Phase 2: What 2026 Price Negotiations Mean for Innovators, Biosimilars, and Global Launch Strategy
The Inflation Reduction Act (IRA) continues to reshape the U.S. pharmaceutical market. After the first list of 10 Medicare Part D drugs entered negotiations in 2023, the clock is now ticking toward Ph ...
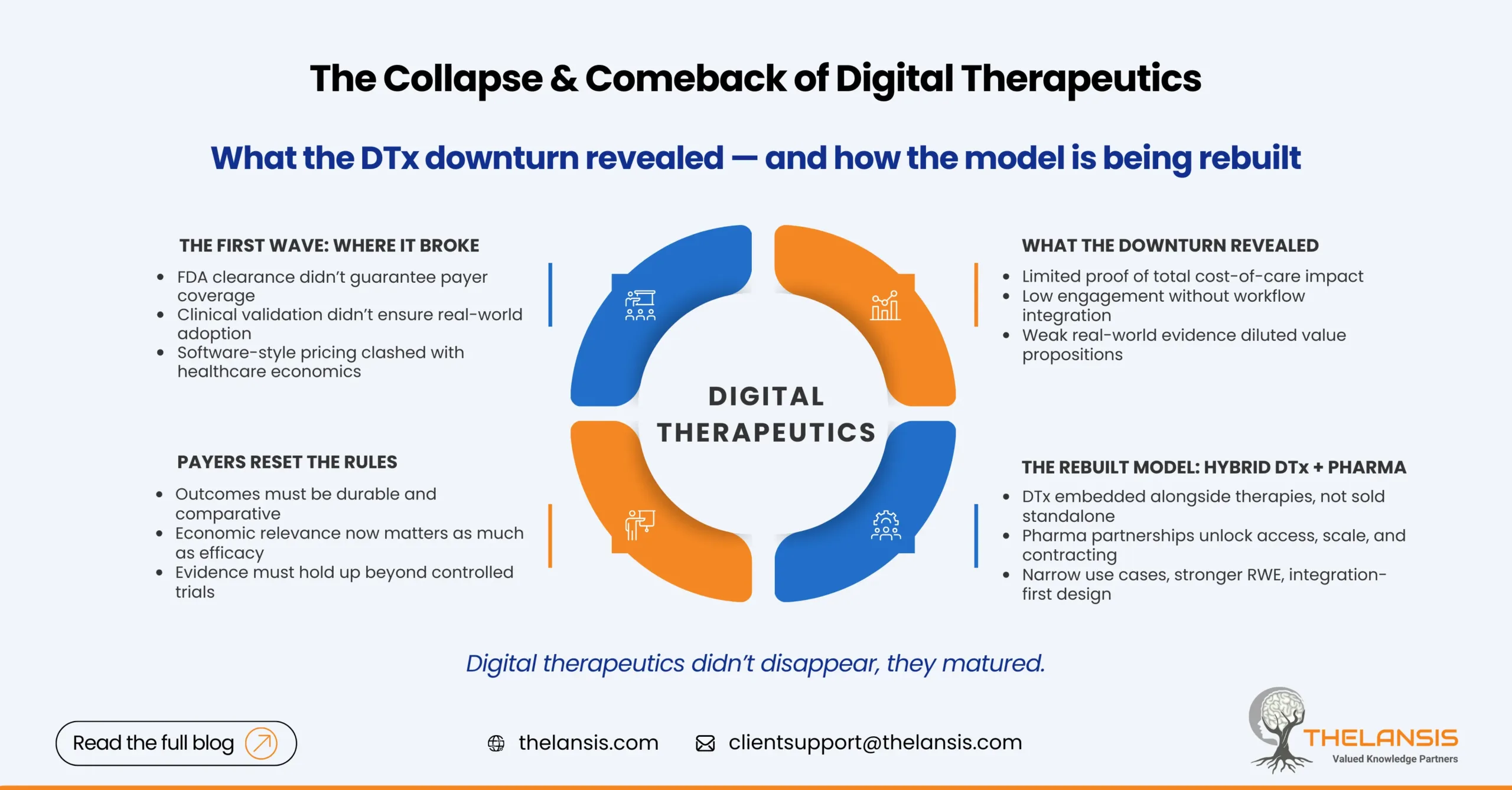
The Collapse & Comeback of Digital Therapeutics: What the Industry Learned and Where It’s Going Next
For a few years, digital therapeutics were treated as inevitable. Prescription apps would scale like software, improve outcomes like medicines, and disrupt chronic care economics along the way. Ventur ...
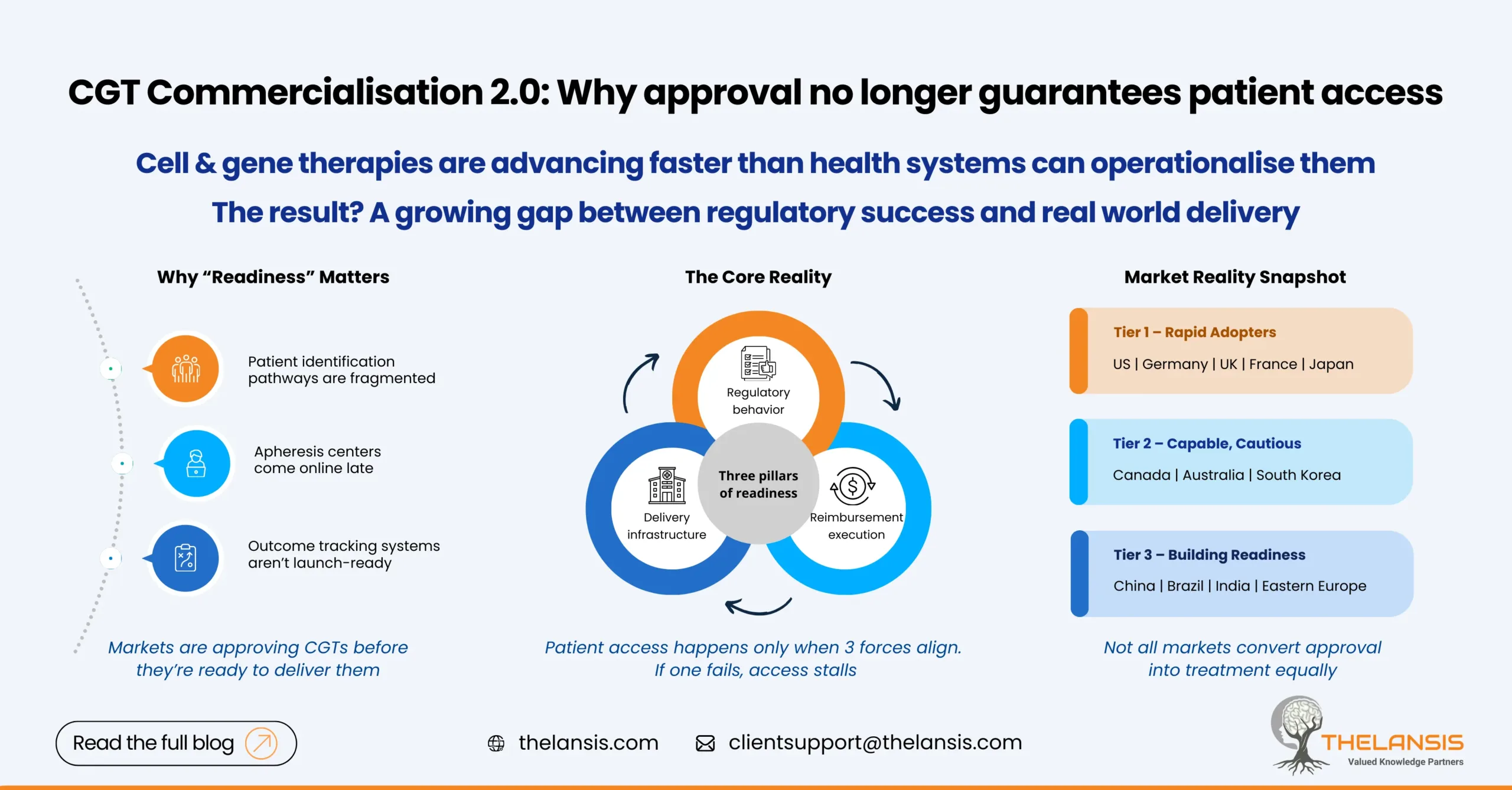
CGT Commercialisation 2.0: Ranking Markets by Regulatory, Infrastructure & Reimbursement Readiness
Cell and gene therapies are no longer a niche category. With more approvals coming through, payers pushing back harder, and health systems struggling to keep up operationally, CGT commercialisation ha ...

Beyond the Drug: Why Ecosystem Based Commercial Models Are Becoming Critical in Rare Diseases
The quiet truth about rare disease medicines is that a brilliant molecule or a one-time gene therapy is only the opening act. Getting the drug from a lab bench miracle into durable, equitable patient ...

Japan’s Evolving HTA Landscape: What Global Launch Teams Must Know
Japan has quietly turned its HTA framework into one of the most influential, but often misunderstood, pricing systems in major markets. Since formal cost-effectiveness evaluation became part of the NH ...
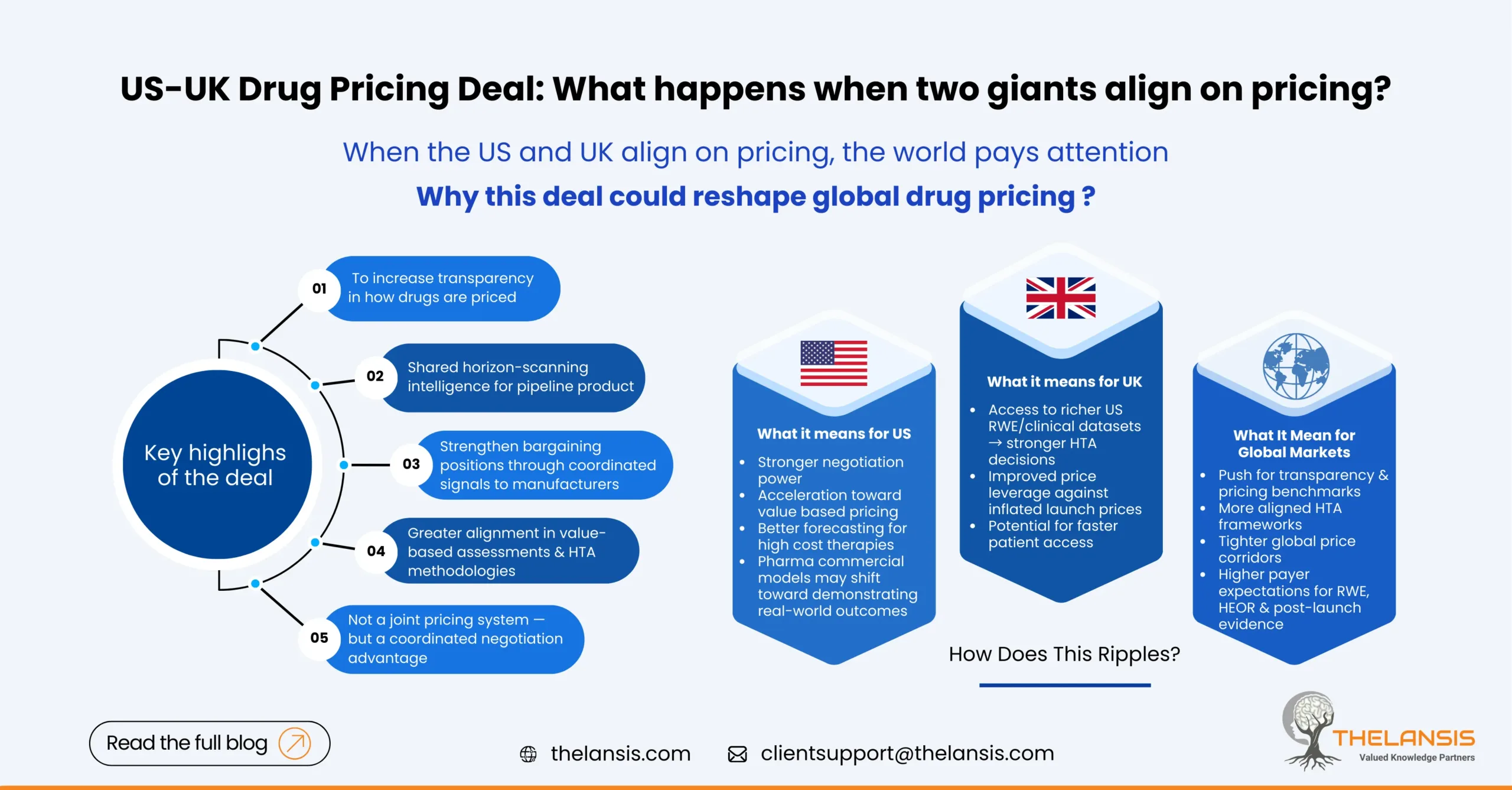
The US-UK Drug Pricing Deal: What It Means for Both Countries and the Global Market
For years, the United States and the United Kingdom have stood on opposite ends of the drug pricing spectrum. The US has historically allowed manufacturers to set list prices with relatively few const ...
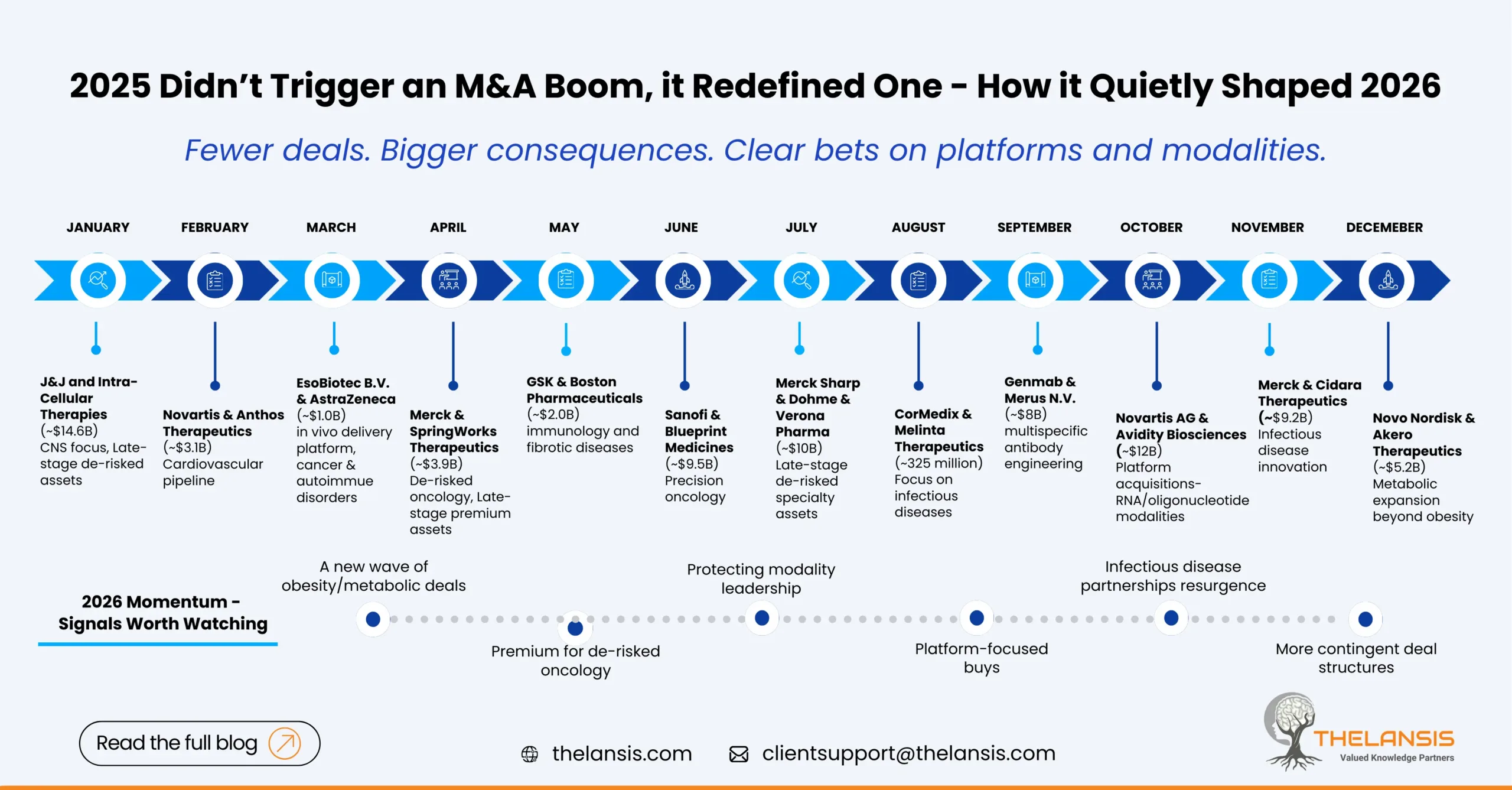
Mergers & Acquisitions in Pharma — What 2025 taught us and where the momentum is heading in 2026
2025 almost felt like pharma pressed a reset button on dealmaking. Money moved in patches, a handful of blockbuster deals grabbed headlines and reshaped competitive positions, while the broader market ...
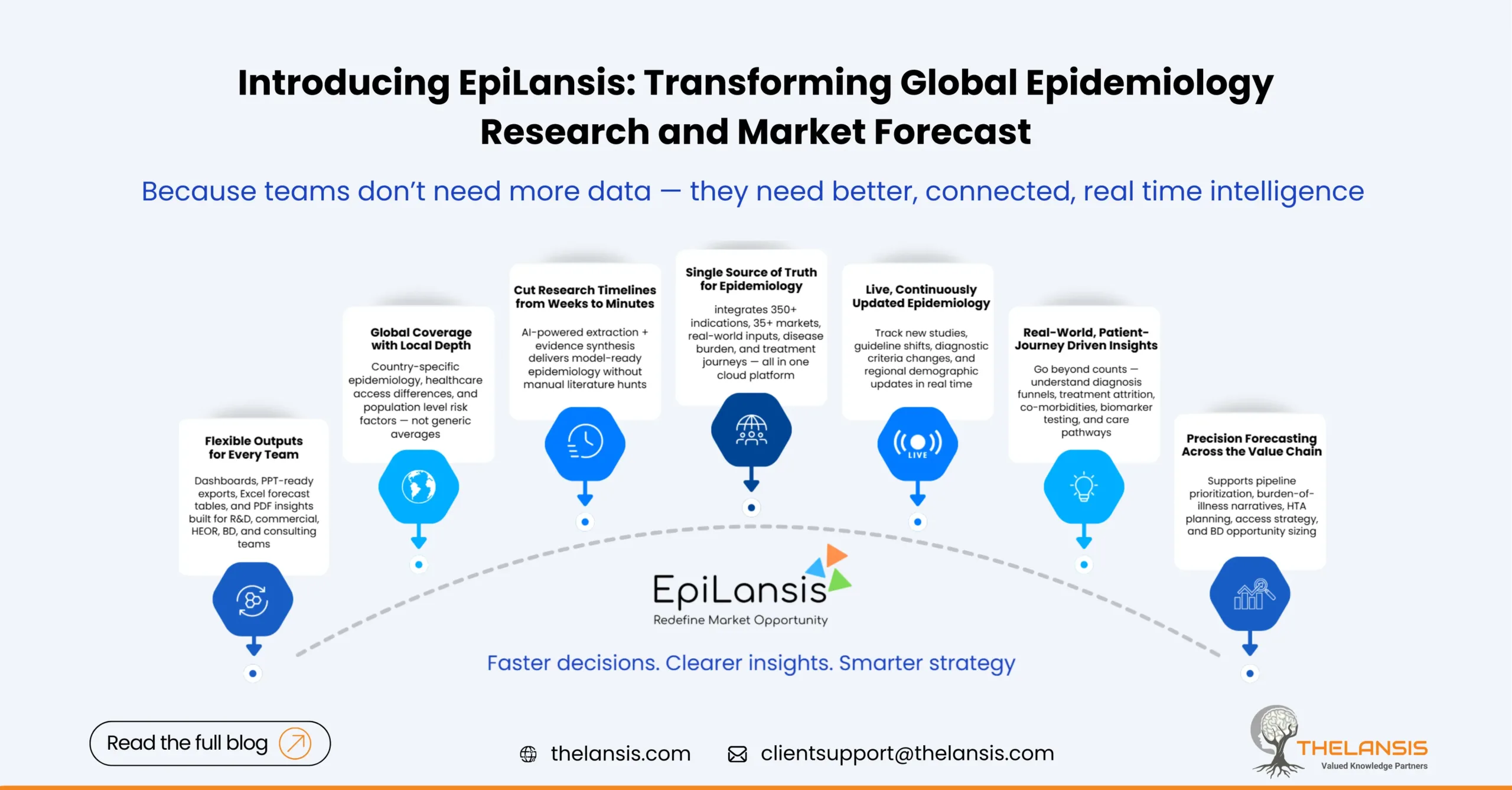
Epilansis: A NextGen, Cloud-based Epidemiology Platform for Faster, Smarter Market Decisions
With the healthcare landscape shifting rapidly, teams across biopharma, consulting, med-tech, and healthcare strategy face a common bottleneck: turning scattered epidemiology data, literature, real wo ...

