
From Data to Drug: How AI Transformed Clinical Trial Outcomes
Introduction: Drug discovery is a complicated, time-intensive, and expensive undertaking, fraught with vast uncertainty. Even with advancements in science and technology, many drug development progra ...

Biohaven’s Taldefgrobep Alfa Receives Fast Track Designation from FDA for Treating Spinal Muscular Atrophy
Biohaven Ltd. has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for its novel anti-myostatin adnectin, taldefgrobep alfa, for treating spinal muscular atrophy (SMA). ...

U.S. FDA Grants Fast Track Designation to Artiva Biotherapeutics’ Lead Program AB-101
Artiva Biotherapeutics, Inc., a clinical-stage company whose mission is to provide highly effective, off-the-shelf, allogeneic natural killer (NK) cell-based therapies, announces that the U.S. FDA has ...

2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 2)
[vc_row css=".vc_custom_1715260973671{margin-top: -30px !important;}"][vc_column][vc_custom_heading text="2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 2)" ...

2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 1)
[vc_row css=".vc_custom_1715260973671{margin-top: -30px !important;}"][vc_column][vc_custom_heading text="2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 1)" ...
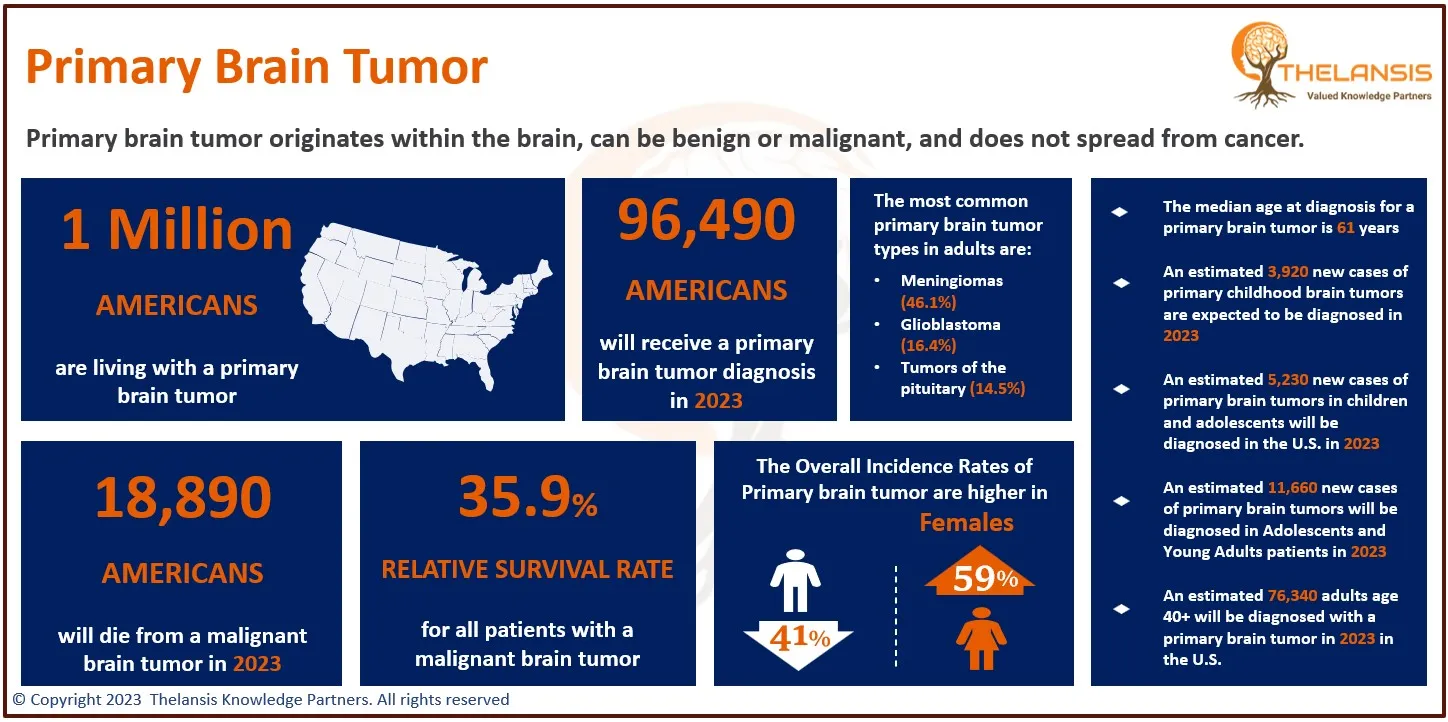
Primary Brain Tumor Overview and Insights
[vc_row][vc_column][vc_custom_heading text="Primary Brain Tumor Overview and Insights" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row][vc_column css=".vc_ ...
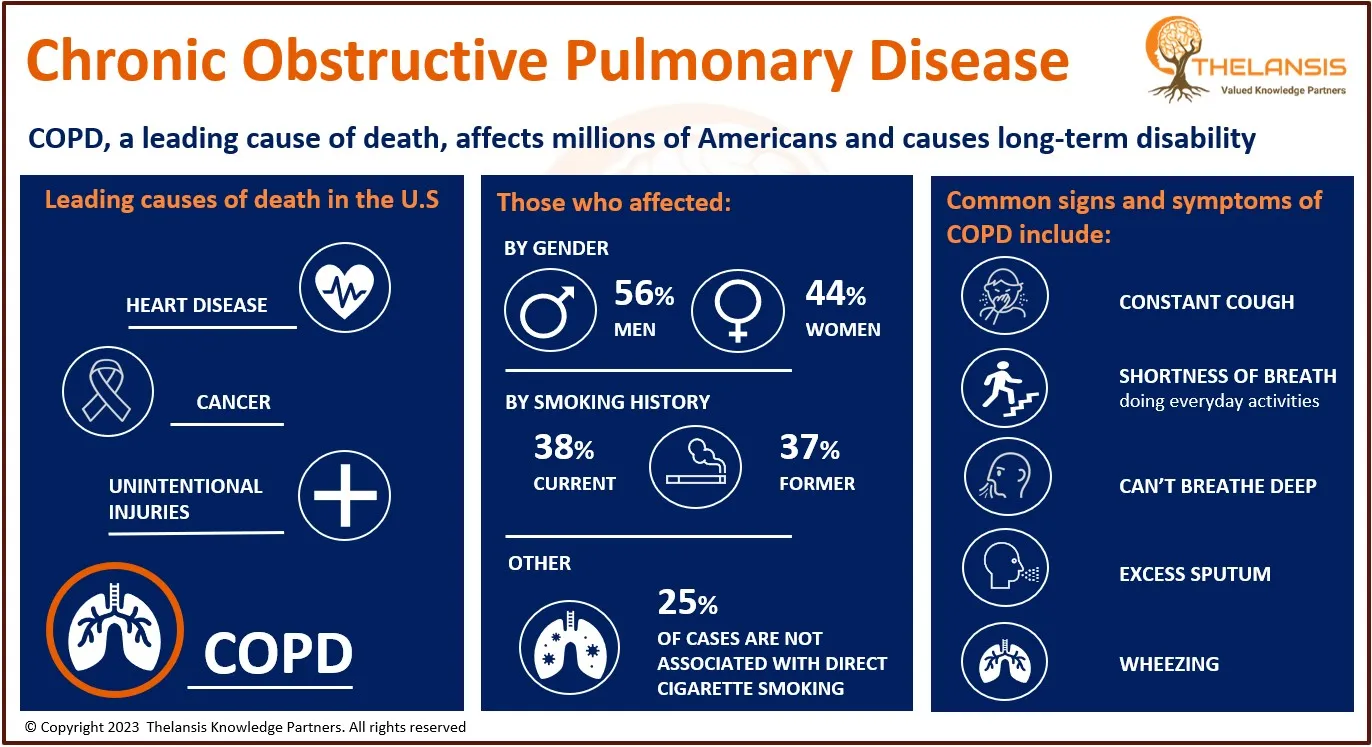
Common Signs and Symptoms of Chronic Obstructive Pulmonary Disease (COPD)
[vc_row][vc_column][vc_custom_heading text="Common Signs and Symptoms of Chronic Obstructive Pulmonary Disease (COPD)" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_ ...


