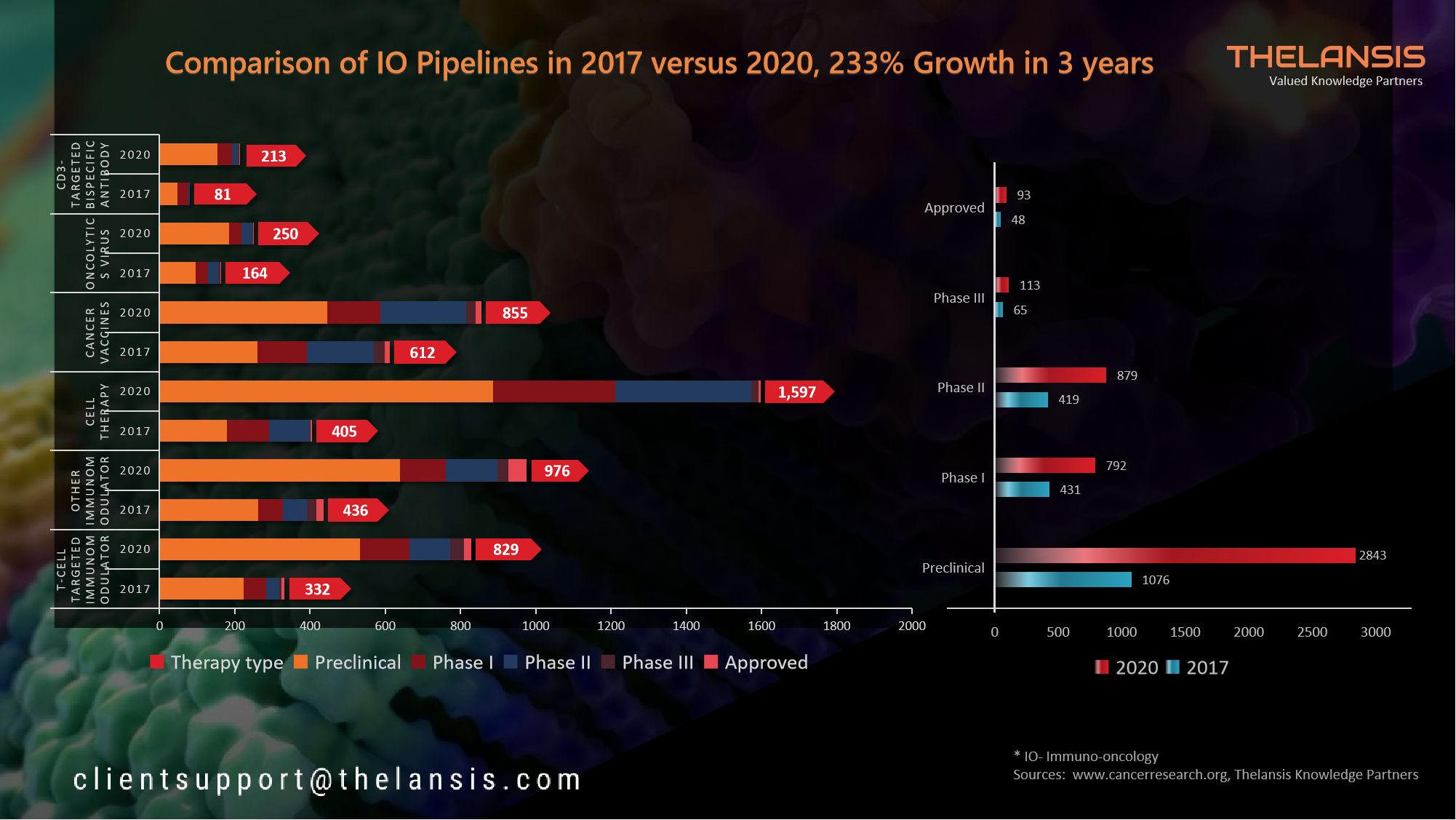
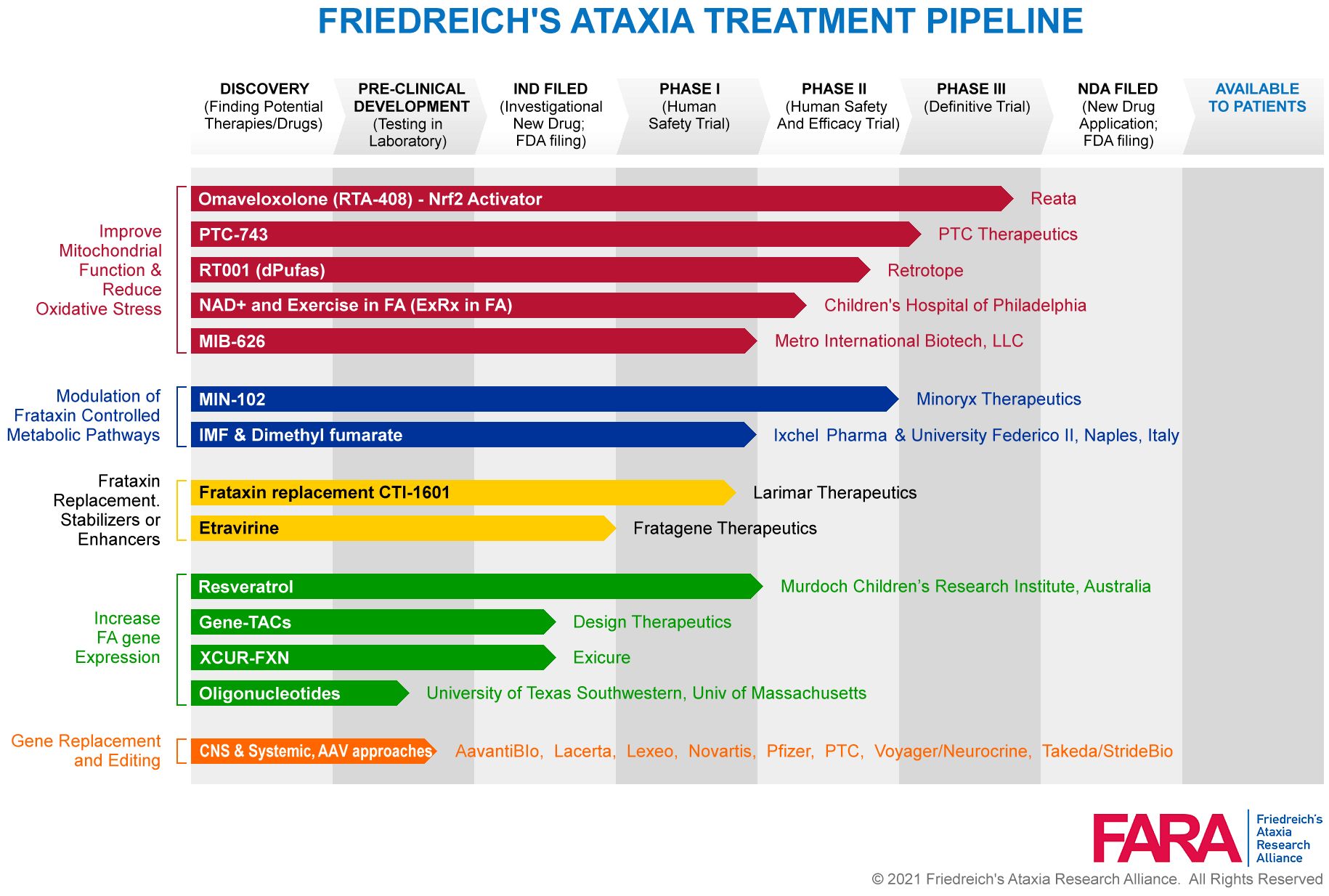
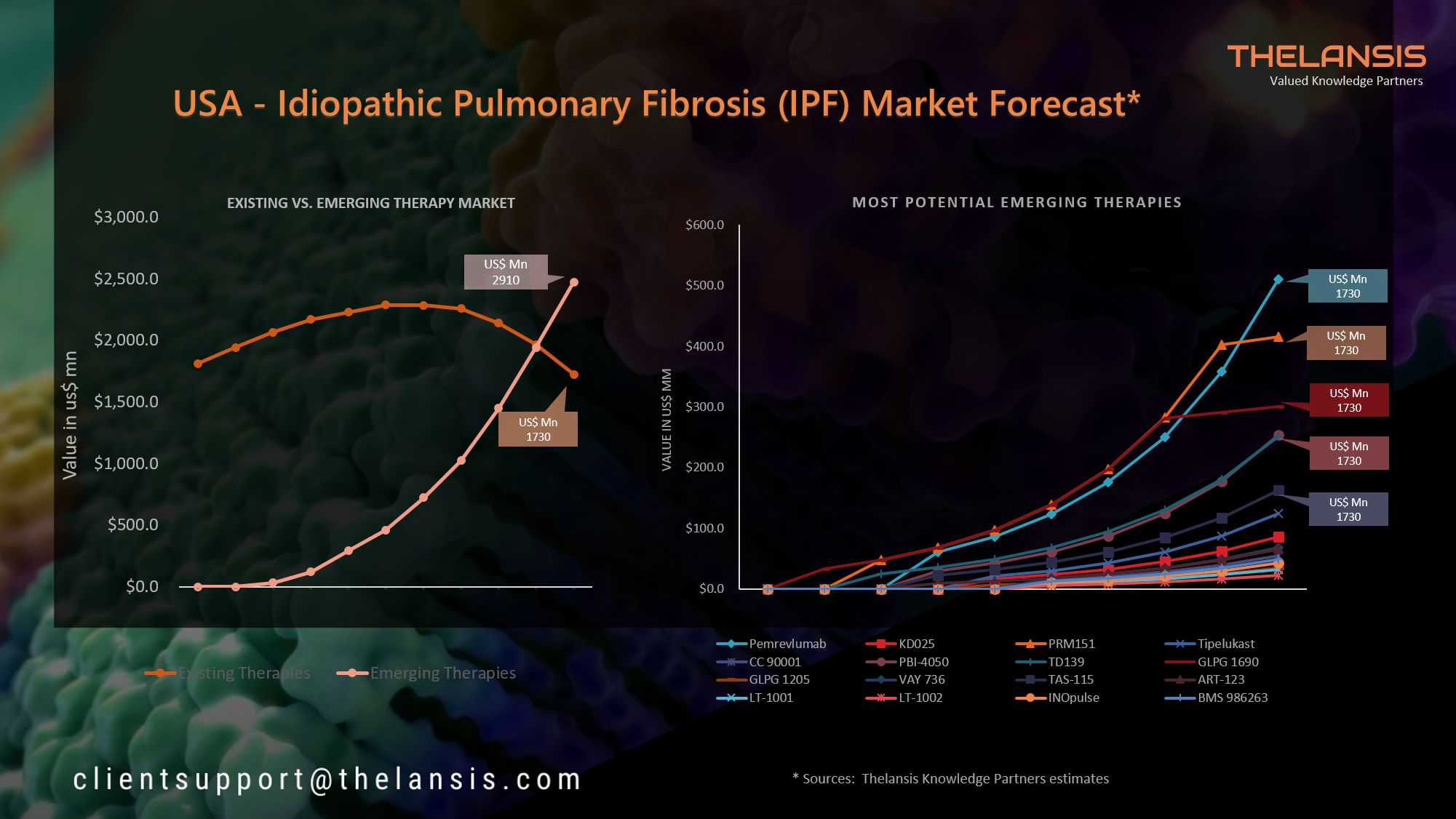
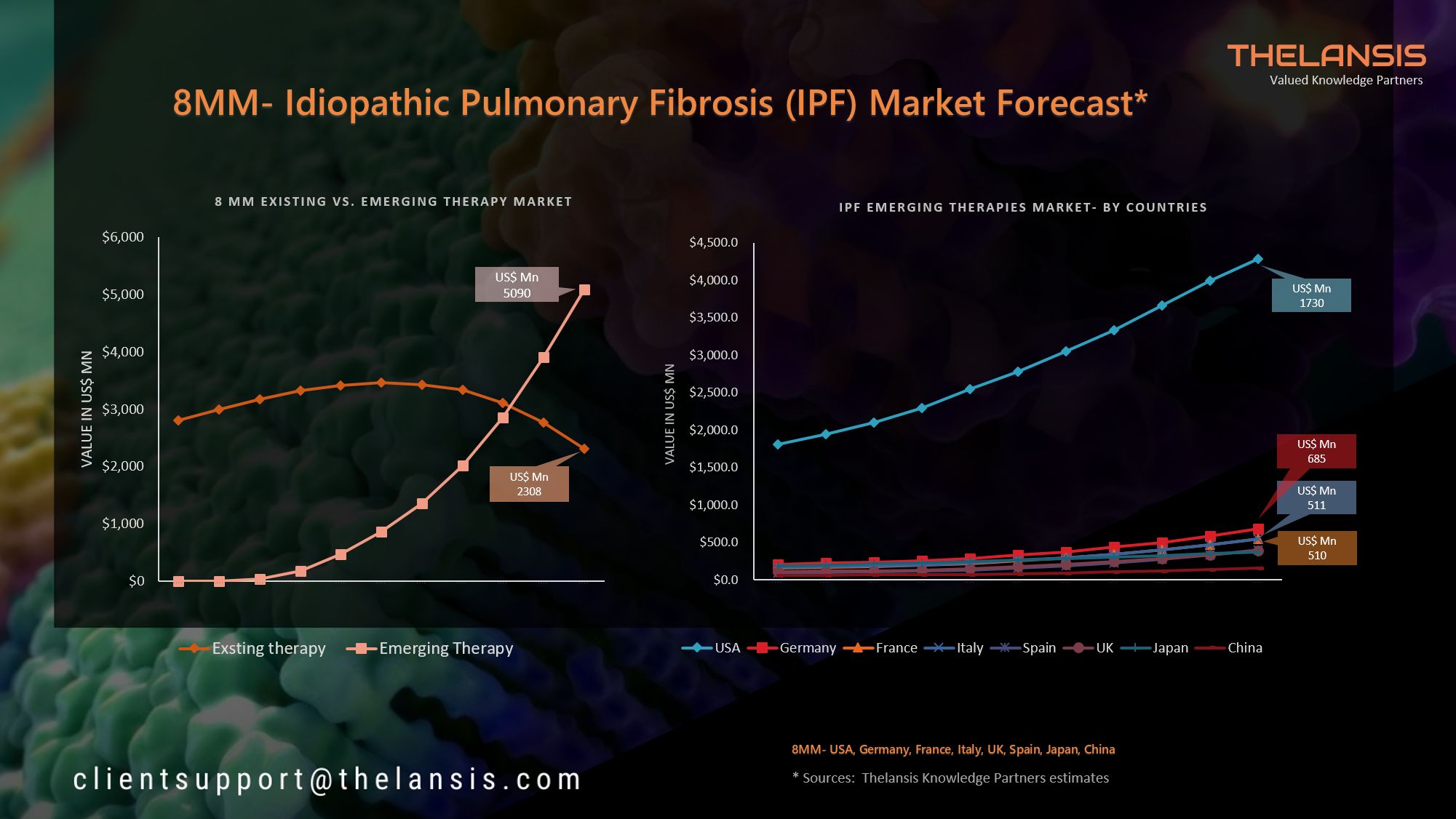
Help your company shift to a digital culture
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...

The ways I can help you
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...

How much does business insurance cost?
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...

Change emails and long video calls for asynchronous video
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...

Personal debt or company debt we explore your options
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...

When is it the right time to sell your company?
Suspendisse potenti nullam ac tortor vitae purus faucibus ornare suspendi sse. Et ultrices neque ornare aenean euismod elementum nisi quis eleifend. Neque egestas congue quisque egestas diam in arcu c ...