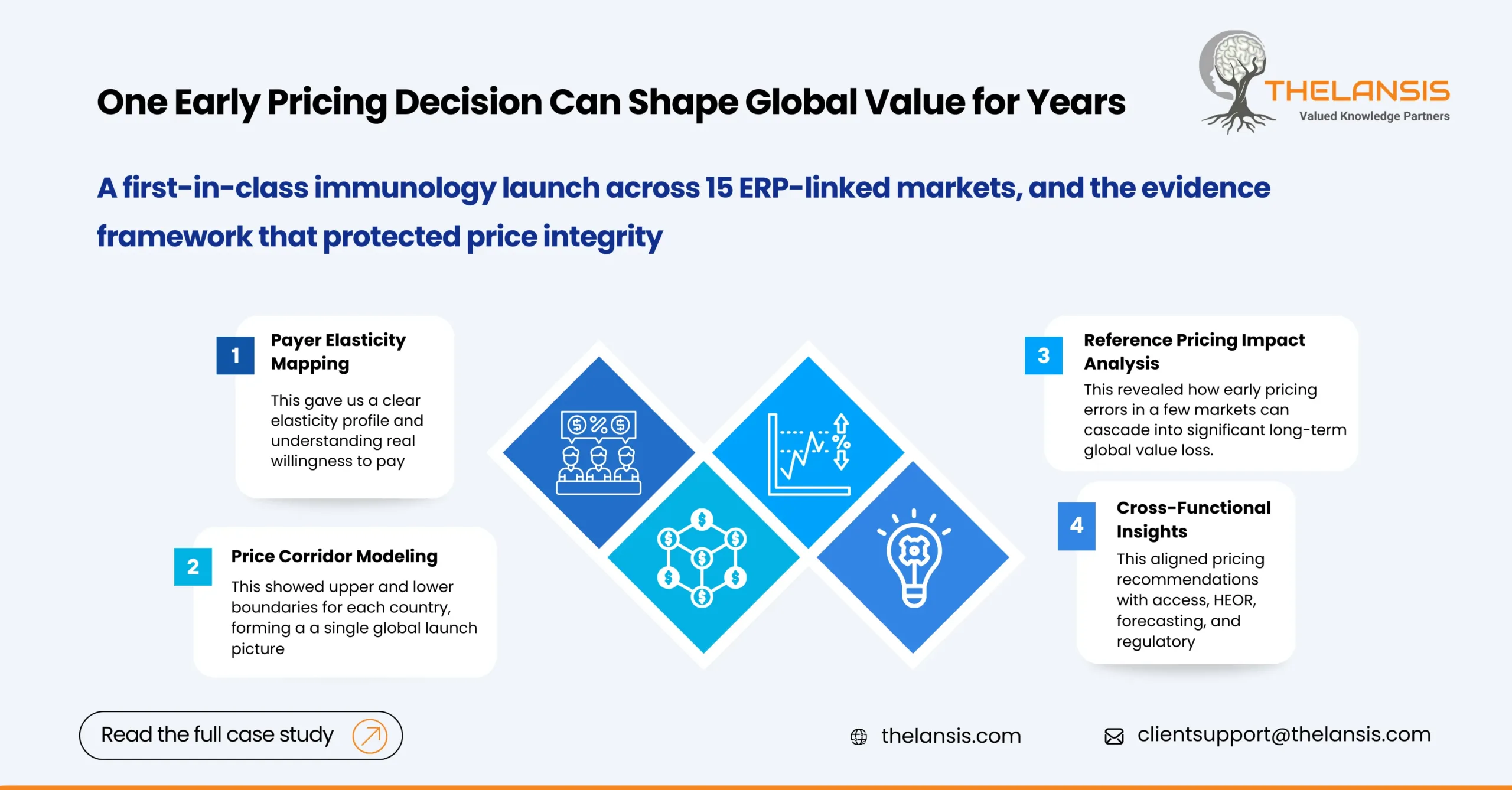
Building a Global Price Strategy: How Thelansis Helped a Biopharma Set the Right Launch Price Across 15 Markets
Background: A biopharma company was preparing to launch a first-in-class therapy in immunology TA. The asset showed strong clinical promise, but its commercial team faced a familiar challenge: every ...
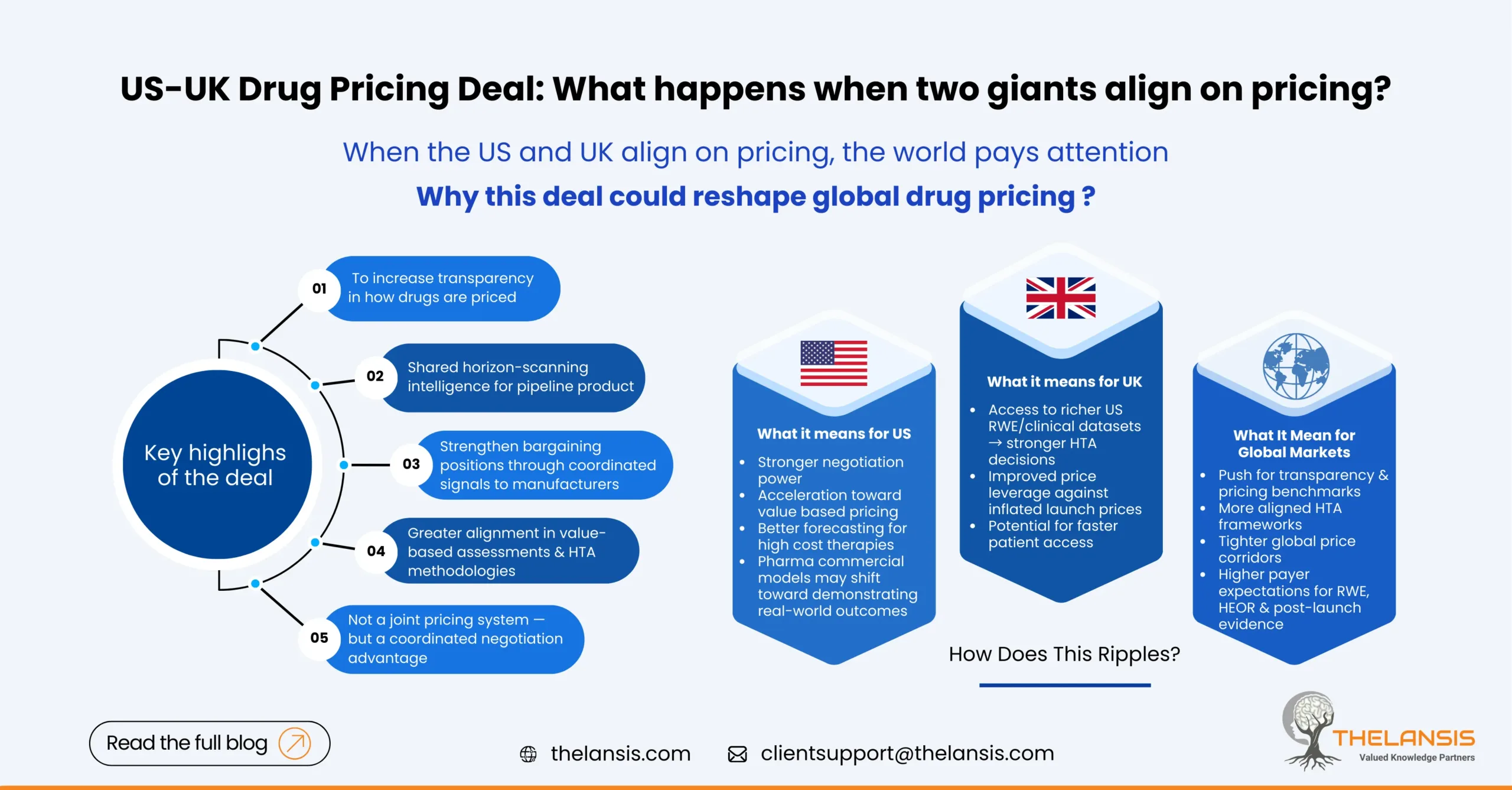
The US-UK Drug Pricing Deal: What It Means for Both Countries and the Global Market
For years, the United States and the United Kingdom have stood on opposite ends of the drug pricing spectrum. The US has historically allowed manufacturers to set list prices with relatively few const ...
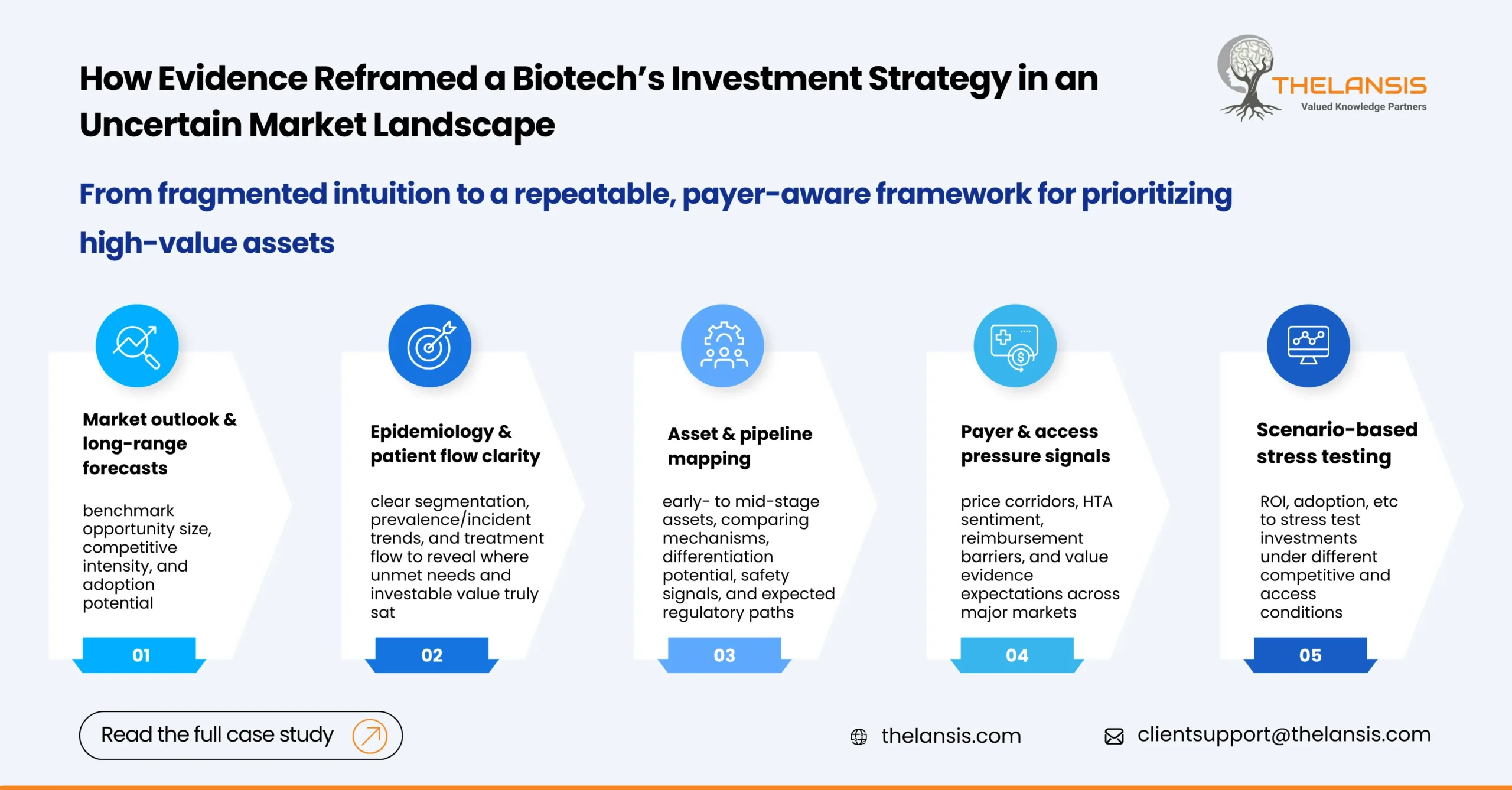
Optimizing Biotech Investment Strategy in an Uncertain Market Landscape
Introduction: A US-based mid-size biotech firm was preparing for its upcoming investment cycle to broaden its therapeutic area focus. The team had strong scientific instincts but lacked a unified, ev ...

Strengthening Global Market Access Readiness Through Early Payer & HTA Planning
Introduction: A global biopharma company preparing to advance towards asset commercialisation approached Thelansis with a clear priority that they wanted to enter multiple markets with confidence tha ...
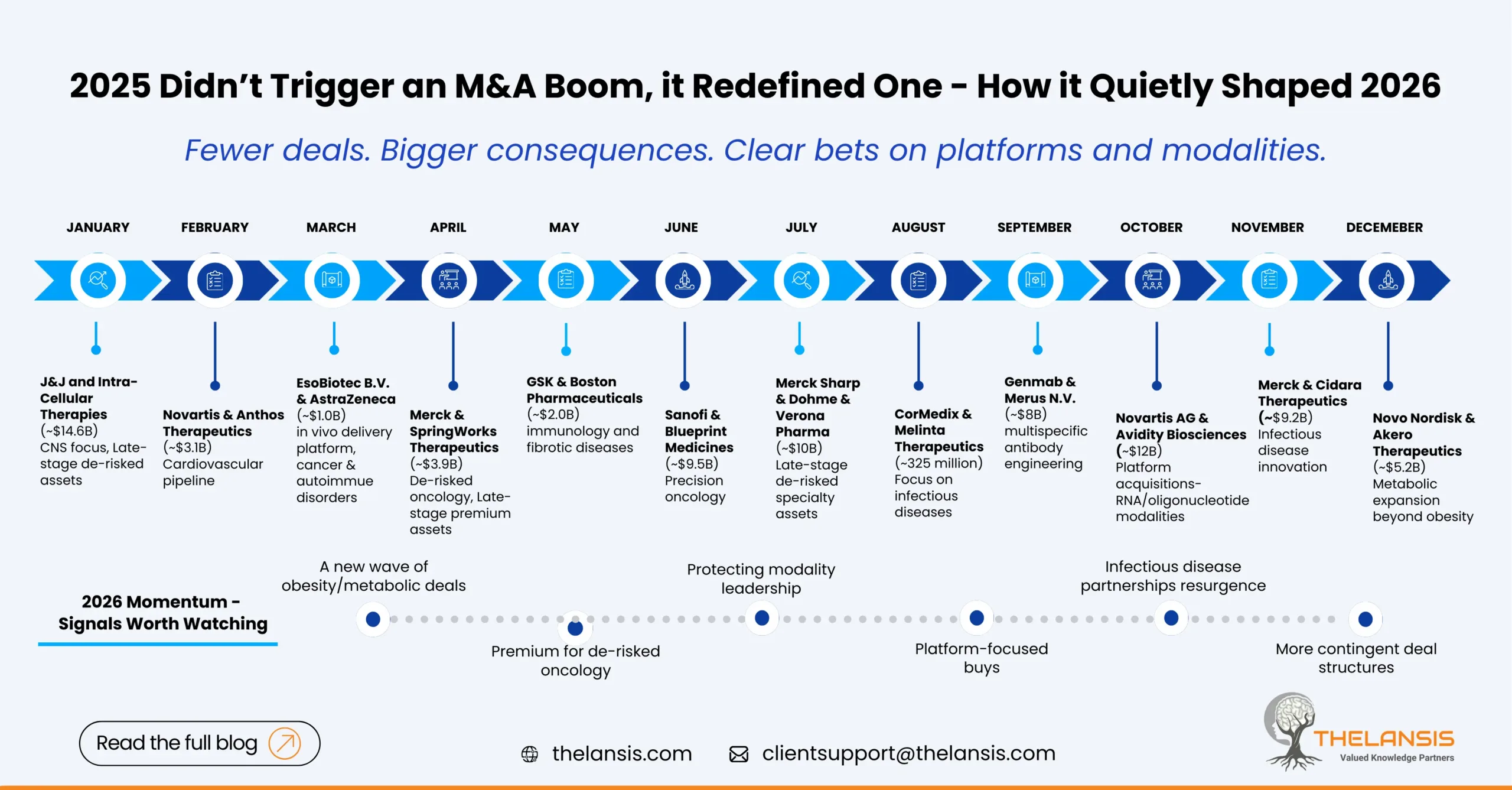
Mergers & Acquisitions in Pharma — What 2025 taught us and where the momentum is heading in 2026
2025 almost felt like pharma pressed a reset button on dealmaking. Money moved in patches, a handful of blockbuster deals grabbed headlines and reshaped competitive positions, while the broader market ...
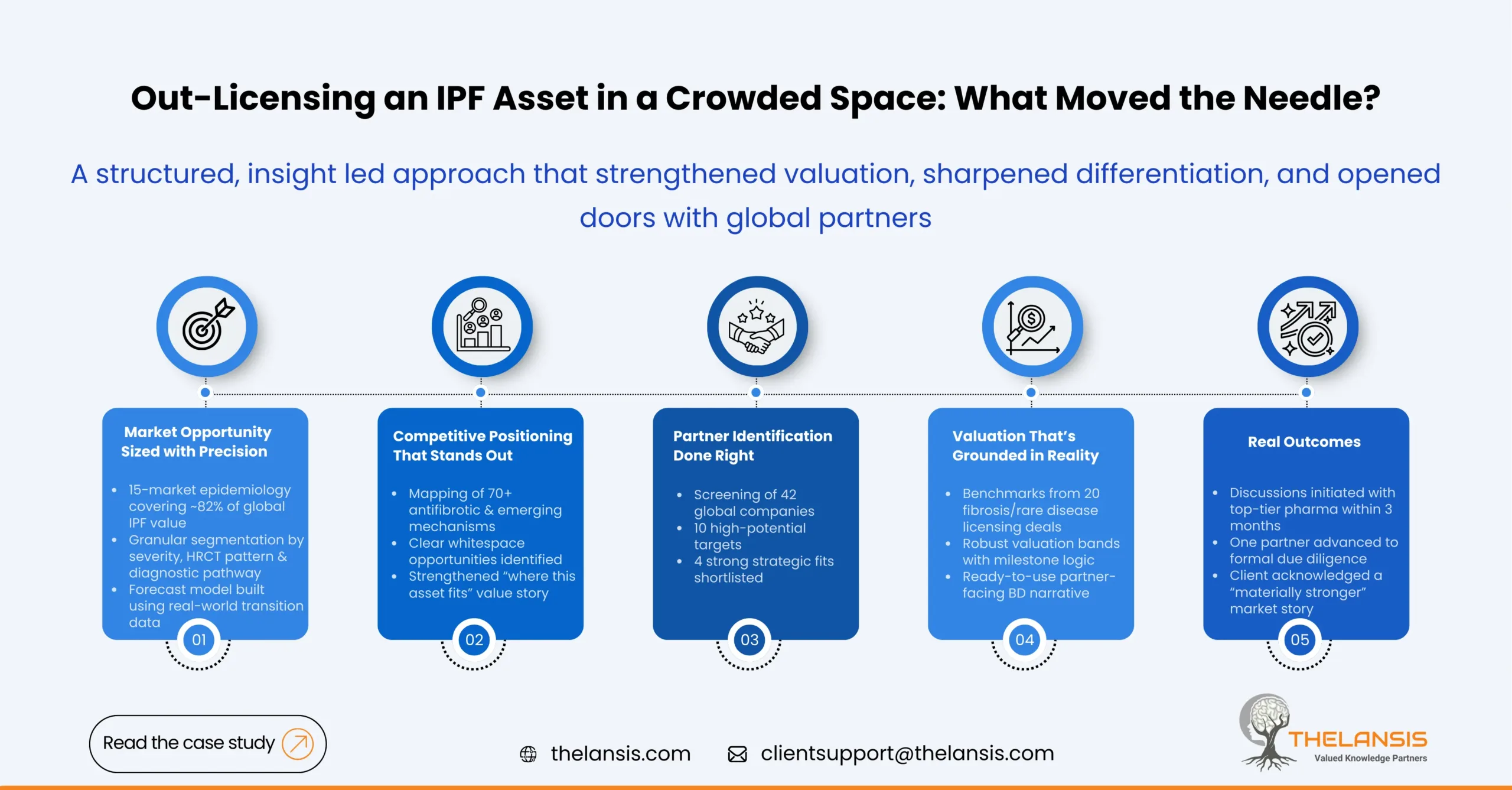
How Thelansis Supported a Mid-Sized Biopharma Company in Out-Licensing Their Novel IPF Asset
Introduction: During 2024, a mid-sized biopharma company from the European region was developing a novel systematic therapy for the treatment of IPF, seeking an evidence-backed structured partnership ...
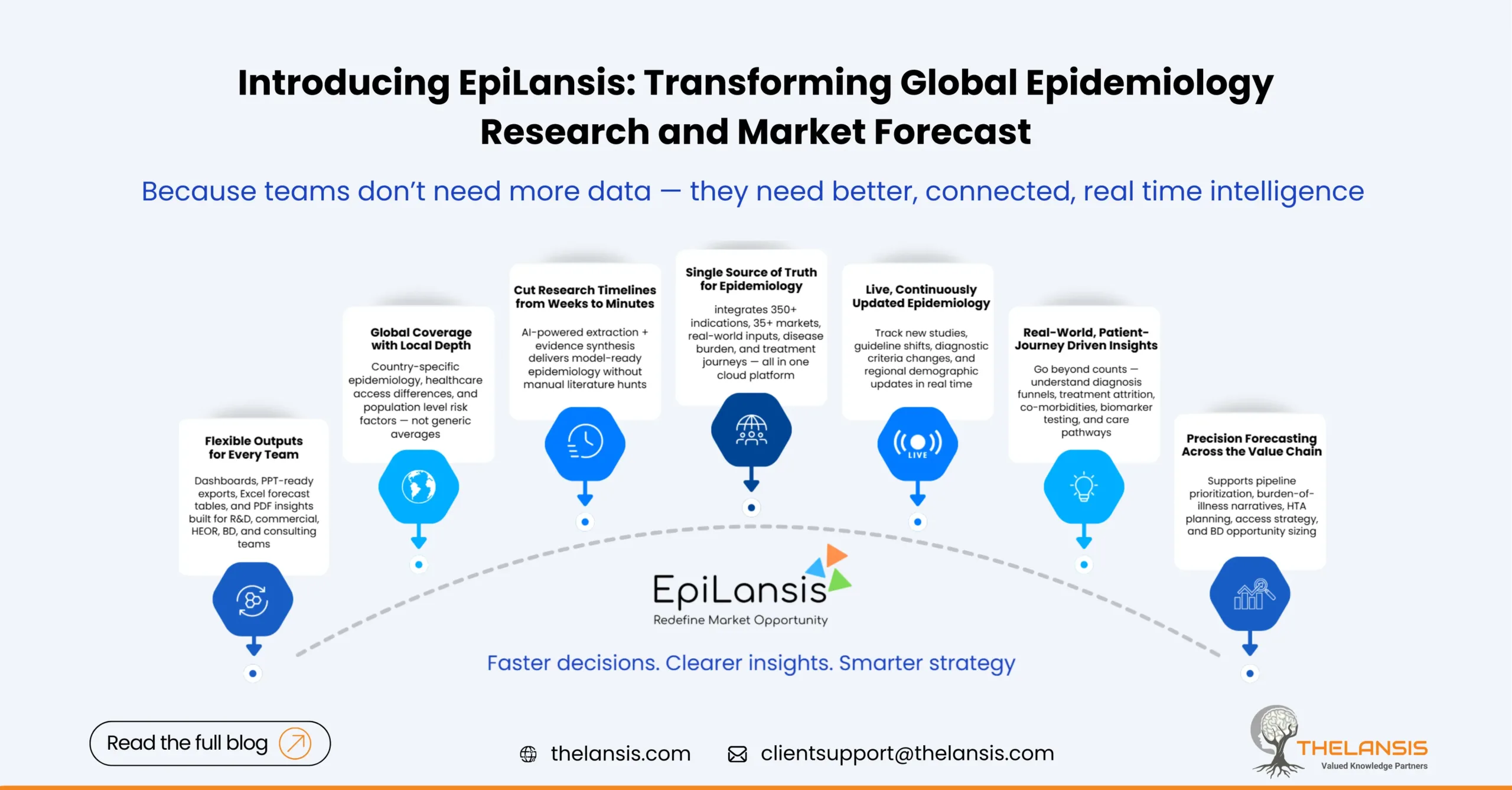
Epilansis: A NextGen, Cloud-based Epidemiology Platform for Faster, Smarter Market Decisions
With the healthcare landscape shifting rapidly, teams across biopharma, consulting, med-tech, and healthcare strategy face a common bottleneck: turning scattered epidemiology data, literature, real wo ...
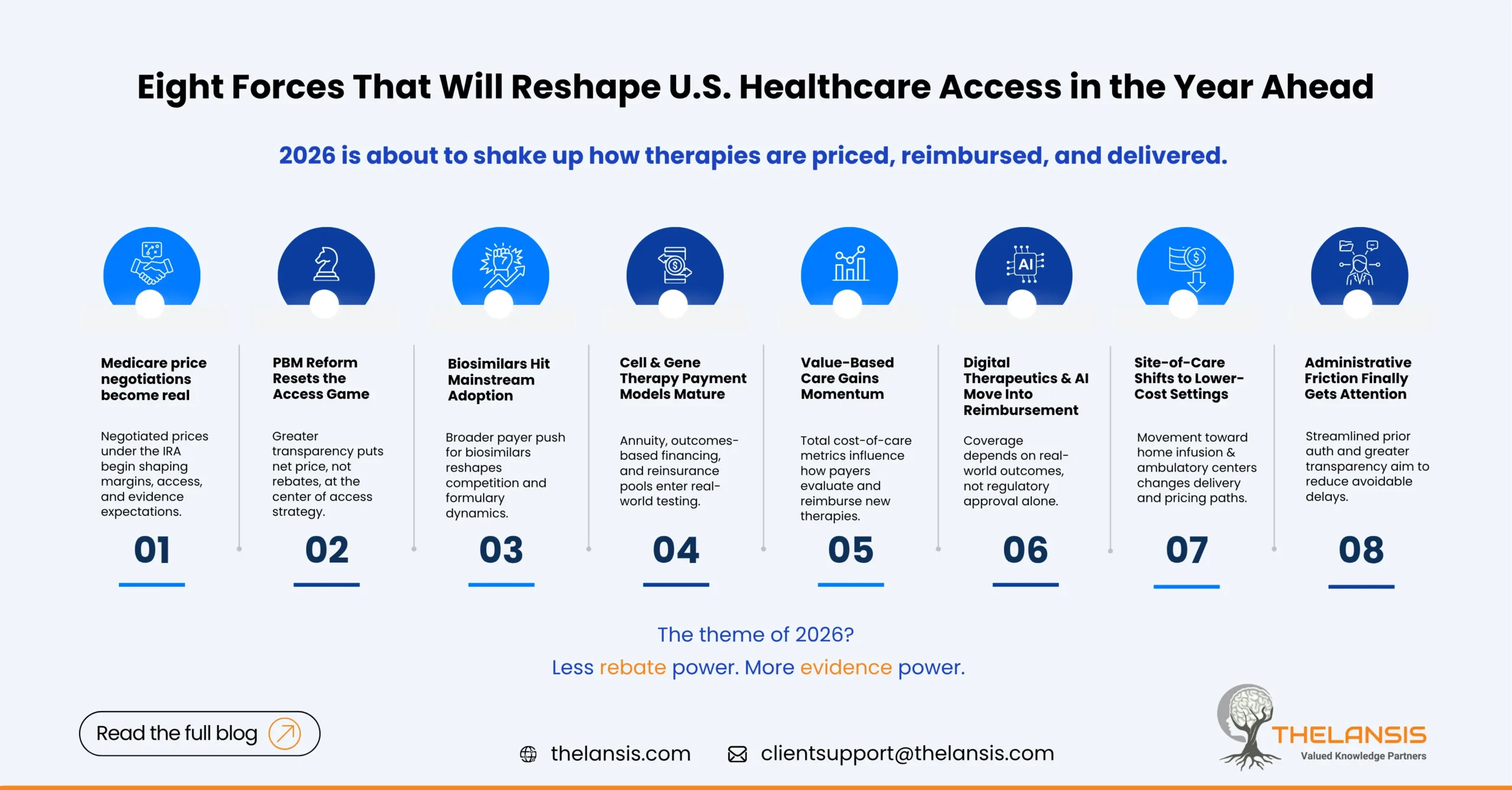
Eight Emerging U.S. Medical Cost & Access Drivers to Watch in 2026
The U.S. healthcare system never really sits still but 2026 is shaping up to be a year where cost, access, and innovation intersect in new and sometimes uncomfortable ways. From the Inflation Reductio ...
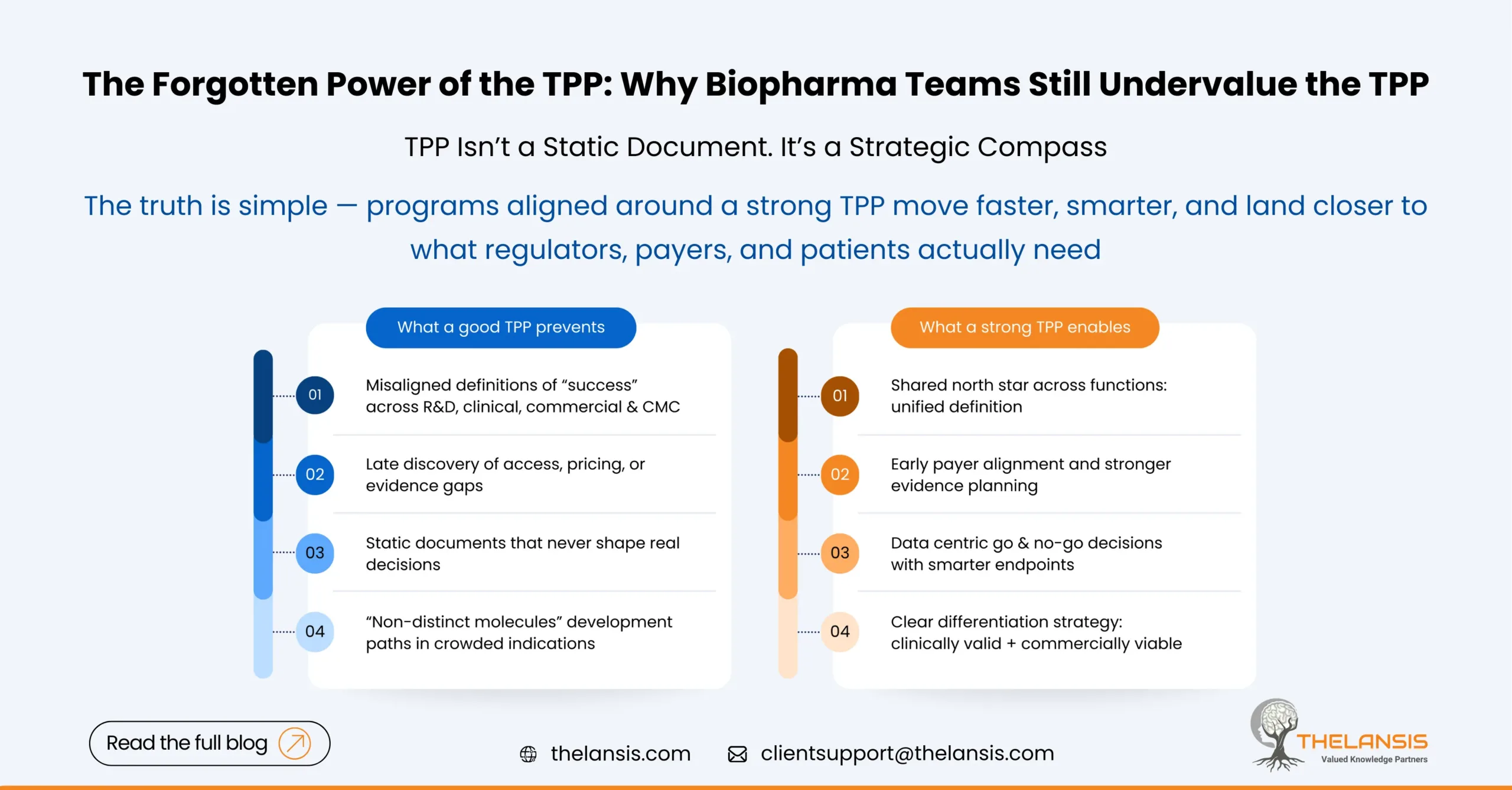
The Forgotten Power of the TPP: Why Many Biopharma Projects Fail Without It
In the fast‐moving world of biopharma innovation, it’s easy to get excited by the science – the novel target, the breakthrough molecule, the cutting-edge modality. But amid that excitement, one strate ...
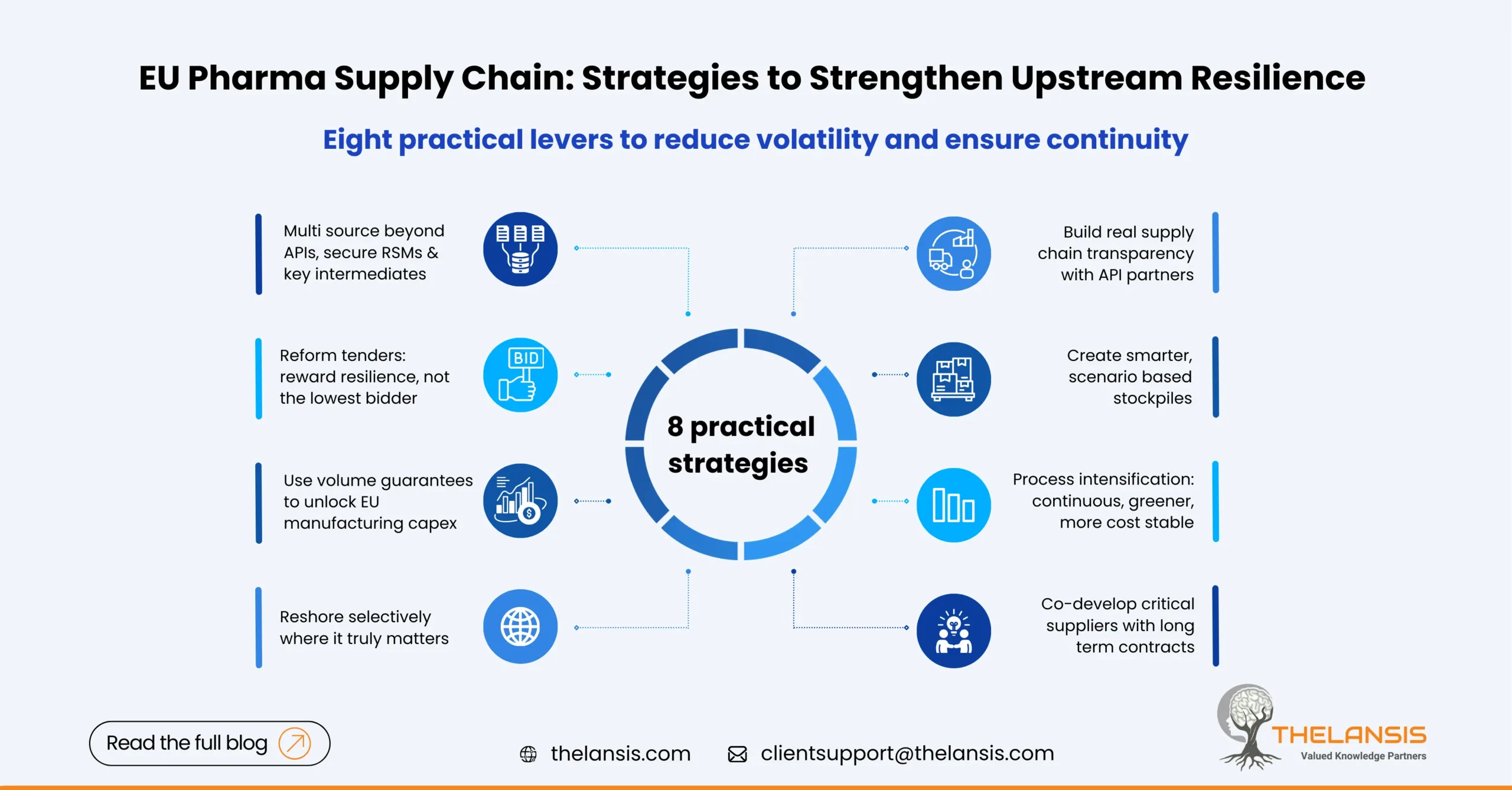
EU Pharmaceutical Supply Chain: How to Shrink Upstream Risk Without Shrinking Access
Europe’s pharmaceutical landscape is undergoing a significant change due to its medicine shortage problem, which has a stubborn root cause: fragile upstream links. The situation becomes apparent at th ...

