
Cullinan Oncology’s CLN-081 Receives Breakthrough Therapy Designation for NSCLC
Cullinan Oncology's CLN-081, an orally available, irreversible EGFR inhibitor, today received an FDA's Breakthrough Therapy Designation to treat locally advanced or metastatic non–small cell lung canc ...

Genprex Receives U.S. FDA Fast Track Designation for REQORSA™ in Combination With Keytruda® for the Treatment of Non-Small Cell Lung Cancer (NSCLC)
Genprex, Inc., today announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track Designation (FTD) for the Company’s lead drug candidate, REQORSA™ Immunogene Therapy, in combinat ...
Dizal’s Sunvozertinib Receives Breakthrough Therapy Designation for First-Line NSCLC Treatment
Dizal, a biopharmaceutical company dedicated to the development of novel treatments for cancer and immunological diseases, has announced that the Center for Drug Evaluation (CDE) of China's National M ...
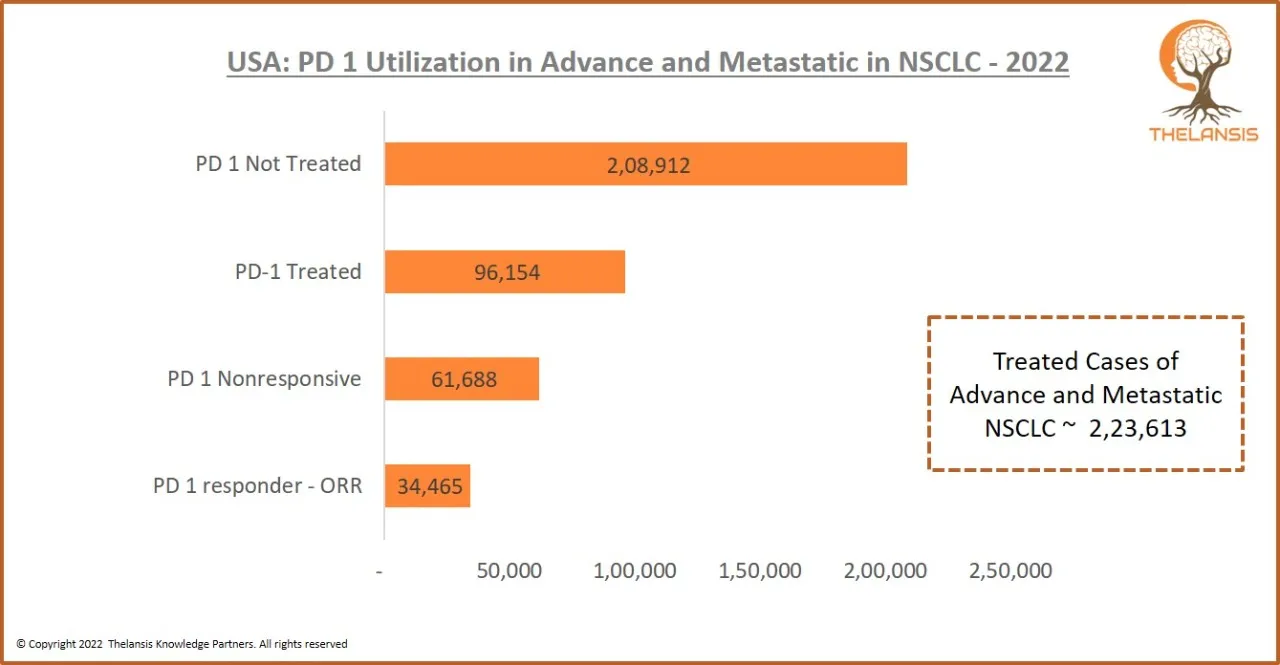
USA: PD 1 Utilization in Advance and Metastatic in NSCLC-2022
[vc_row][vc_column][vc_custom_heading text="USA: PD 1 Utilization in Advance and Metastatic in NSCLC-2022" font_container="tag:h2|text_align:center" use_theme_fonts="yes"][/vc_column][/vc_row][vc_row] ...

