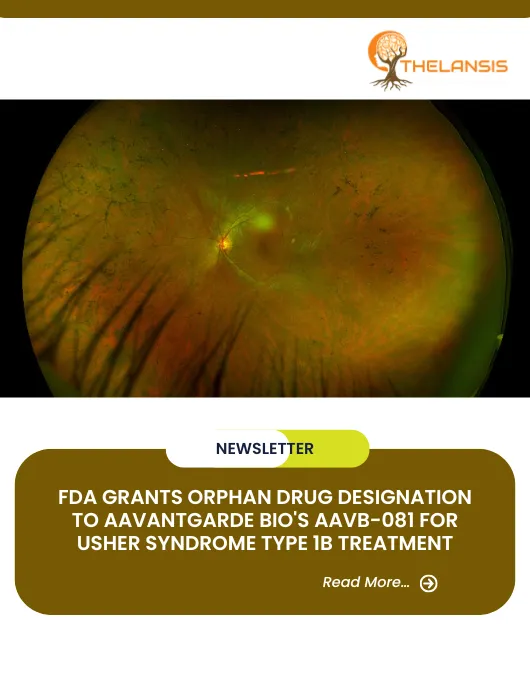
FDA Grants Orphan Drug Designation to AAVantgarde Bio’s AAVB-081 for Usher Syndrome Type 1B Treatment
FDA Grants Orphan Drug Designation to AAVantgarde Bio's AAVB-081 for Usher Syndrome Type 1B Treatment AAVantgarde Bio (AAVantgarde), a clinical-stage, Italian-based international biotechnology company ...
Ractigen Therapeutics’ RAG-21 Receives FDA Orphan Drug Designation for Treating FUS-ALS
Ractigen Therapeutics' RAG-21 Receives FDA Orphan Drug Designation for Treating FUS-ALS Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing innovative therapies, tod ...
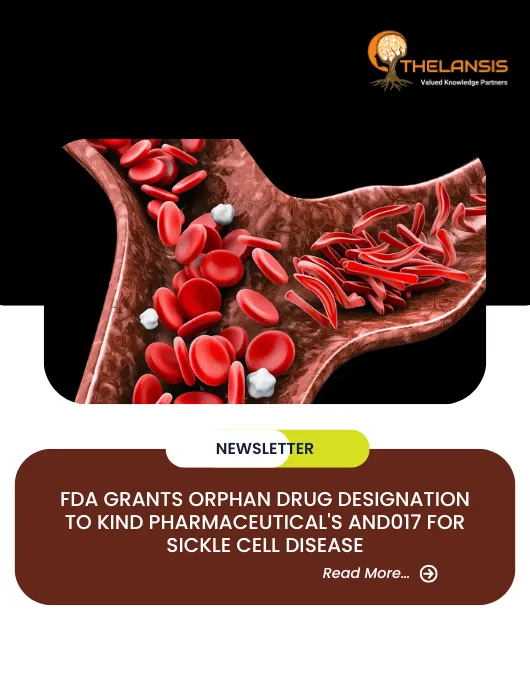
FDA Grants Orphan Drug Designation to Kind Pharmaceutical’s AND017 for Sickle Cell Disease
Kind Pharmaceutical, a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, announced that the U.S. Food and Drug Administra ...

