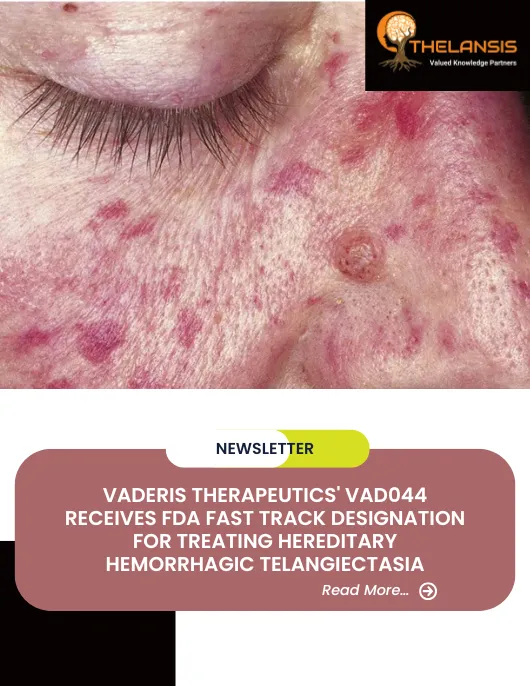
Vaderis Therapeutics’ VAD044 Receives FDA Fast Track Designation for Treating Hereditary Hemorrhagic Telangiectasia
Vaderis Therapeutics' VAD044 Receives FDA Fast Track Designation for Treating Hereditary Hemorrhagic Telangiectasia Vaderis Therapeutics AG, a clinical-stage biotechnology company focusing on treatmen ...

FDA Grants Fast Track Designation to Valneva and LimmaTech’s Shigella4V Vaccine
Valneva SE, a specialty vaccine company, and LimmaTech Biologics AG, a clinical-stage biotech firm focused on preventing life-threatening diseases, have announced that the U.S. Food and Drug Administr ...
FDA Grants Fast Track Designation to Lantern Pharma’s AI-Enhanced Drug LP-184 for Glioblastoma
Lantern Pharma Inc., an AI company pioneering cancer therapies, announces that the FDA has granted Fast Track Designation for its investigational drug, LP-184, to treat Glioblastoma. Currently in a Ph ...
