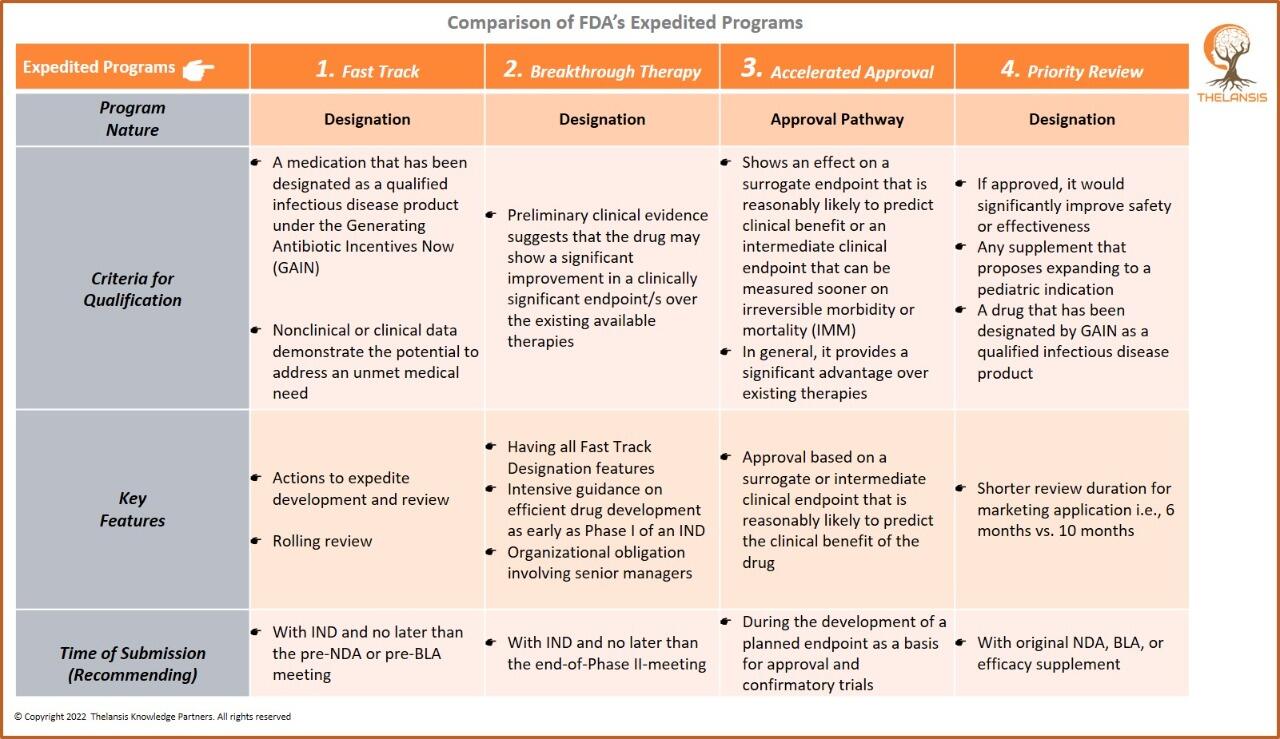
Biohaven’s Taldefgrobep Alfa Receives Fast Track Designation from FDA for Treating Spinal Muscular Atrophy
Biohaven Ltd. has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for its novel anti-myostatin adnectin, taldefgrobep alfa, for treating spinal muscular atrophy (SMA). ...