SpliSense Announces EMA and FDA Grant Orphan Drug Designation to SPL84-23-1 for the Cystic Fibrosis Treatment
SpliSense, a biopharmaceutical company developing transformative mRNA-altering therapies for cystic fibrosis (CF) and other pulmonary diseases, today announced that the U.S. Food and Drug Administrati ...
Vir Biotechnology Secures EMA Orphan Drug Designation for Tobevibart and Elebsiran in Treating Chronic Hepatitis Delta
Vir Biotechnology Secures EMA Orphan Drug Designation for Tobevibart and Elebsiran in Treating Chronic Hepatitis Delta Vir Biotechnology, Inc. has announced a significant milestone in the fight agains ...

EMA Grants Orphan Drug Designation to NIDO-361 for Kennedy’s Disease
Nido Biosciences, a biopharmaceutical company developing medicines to treat debilitating neurological diseases with precision, today announced that the European Medicines Agency has granted an Orphan ...
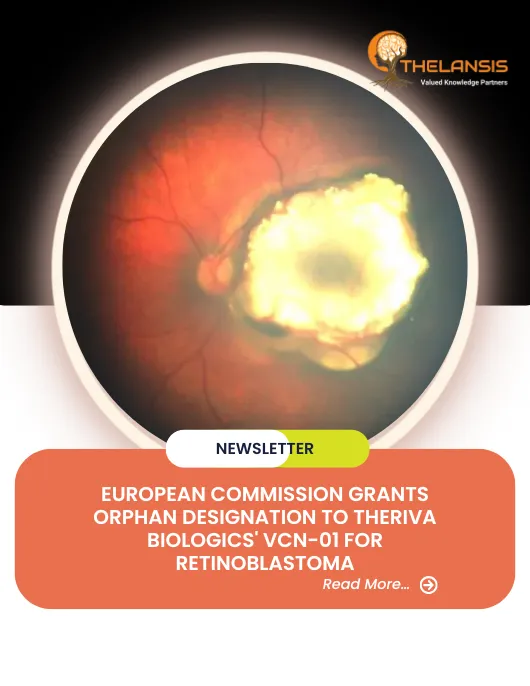
European Commission Grants Orphan Designation to Theriva Biologics’ VCN-01 for Retinoblastoma
Theriva Biologics, a clinical-stage biotech company developing cancer therapeutics, announced that the European Commission has adopted the EMA's recommendation to grant orphan medicinal product design ...

