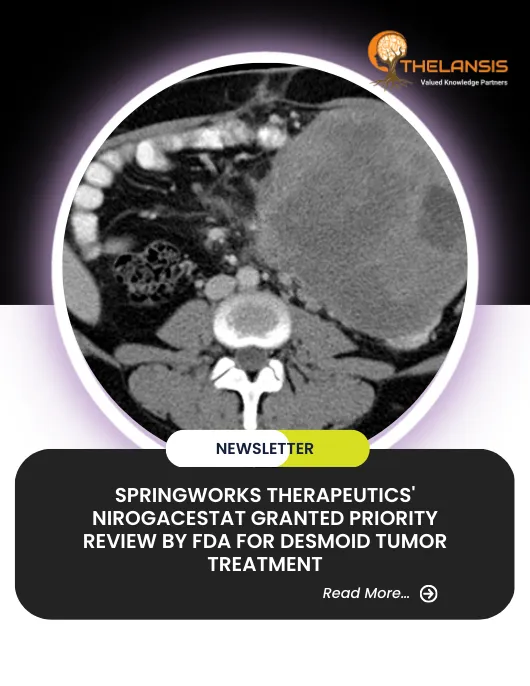
SpringWorks Therapeutics’ Nirogacestat Granted Priority Review by FDA for Desmoid Tumor Treatment
SpringWorks Therapeutics, a clinical-stage biopharmaceutical company, has received acceptance from the U.S. Food and Drug Administration (FDA) for its New Drug Application (NDA) for nirogacestat, an i ...
IASO Bio’s CT103A Receives RMAT & Fast Track Designation from FDA for Treating Relapsed/Refractory Multiple Myeloma
IASO Biotherapeutics, a clinical-stage biopharmaceutical company, today announced that the United States Food and Drug Administration (FDA) has granted both Regenerative Medicine Advanced Therapy (RMA ...
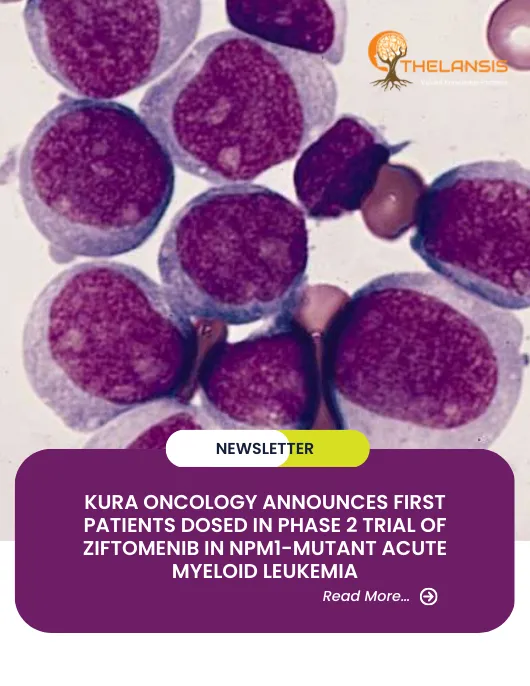
Kura Oncology Announces First Patients Dosed in Phase 2 Trial of Ziftomenib in NPM1-Mutant Acute Myeloid Leukemia
Kura Oncology Inc., a clinical-stage biopharmaceutical company dedicated to realizing the promise of precision medicines for cancer treatment, announced today that the first patients in its KOMET-001 ...

FDA Grants Orphan Drug Designation to Ezurpimtrostat (GNS561) for Hepatocellular Carcinoma (HCC) Treatment
Genoscience Pharma today announces that its lead candidate, ezurpimtrostat (GNS561), a PPT-1 (Palmitoyl Protein Thioesterase-1) inhibitor, has been granted Orphan Drug Designation by the U.S. FDA for ...

FDA Grants Orphan Drug Status to Beactica’s BEA-17 for Glioblastoma Treatment
Beactica Therapeutics, a Swedish precision oncology company, announced today that the U.S. FDA has designated BEA-17 as an orphan drug for the treatment of glioblastoma (GBM). BEA-17 is a first-of-its ...

DualityBio’s DB-1303 Granted Fast-Track Status by FDA for Advanced Endometrial Cancer Treatment
Duality Biologics has announced that the FDA has granted Fast Track Designation to DB-1303, a novel antibody-drug conjugate, for the treatment of advanced, recurrent or metastatic endometrial carcinom ...

