May 17 2023
/
XORTX Therapeutics Secures Orphan Drug Designation for Oxypurinol to Treat Autosomal Dominant Polycystic Kidney Disease
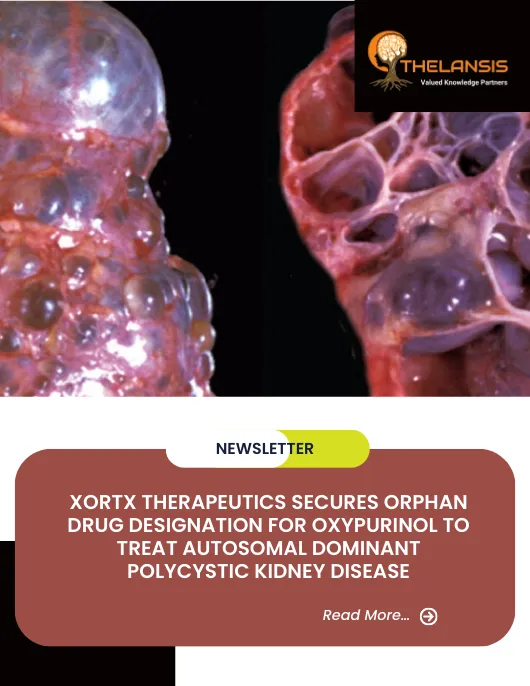
XORTX Therapeutics Inc., a late-stage clinical pharmaceutical company that aims to create new therapies to treat progressive kidney disease, is excited to announce that they have received Orphan Drug Designation for their drug, oxypurinol. This designation has been granted for the treatment of autosomal dominant polycystic kidney disease (ADPKD).
Publish Date: 21-04-2023 Source: XORTX Therapeutics Inc.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disorder caused by mutations in ADPKD1 or ADPKD2 genes. ADPKD1 gene mutations account for approximately 85 percent of cases; ADPKD2 gene mutations account for roughly 15 percent of cases. ADPKD1 is the most severe form of the disease; ADPKD2 patients develop kidney insufficiency about 20 years later. These mutations are passed down through families as an autosomal dominant trait. In about 10% of cases, the mutation occurs randomly and for no apparent reason (sporadically). ADPKD1 gene mutations are generally associated with more severe disease, an earlier age of onset, and an earlier age of onset of end-stage renal disease. The specific symptoms and their severity can vary significantly from person to person, even within the same family. Most affected people develop symptoms between the third and fifth decades of life. On the other hand, symptoms can appear during childhood or even infancy. The course is characterized by the development and inexorable expansion of multiple cysts scattered throughout the kidney parenchyma. The progressive loss of kidney function takes place over many decades and frequently leads to end-stage kidney disease during or after the sixth decade of life.
- ADPKD has an estimated prevalence of 40 to 48 cases per 100,000 population in the USA.
However, the current Autosomal dominant polycystic kidney disease (ADPKD) treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Tolvaptan, Bardoxolone methyl, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the ADPKD treatment. The key companies in the advanced development stage are Otsuka Pharmaceutical Development & Commercialization, Inc., Reata Pharmaceuticals, Inc., etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Autosomal dominant polycystic kidney disease (ADPKD) to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2021 to 2032.
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com - Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com
Related posts:
- Atriva receives FDA Orphan Drug Designation for Zapnometinib to treat Hantavirus Infections
- FDA Grants Orphan Drug Status to Zydus Lifesciences’ ZYIL1 for Treating Cryopyrin-Associated Periodic Syndrome
- Sumitomo Pharma’s TP-1287 granted FDA Orphan Drug and Rare Pediatric Disease Designations for Ewing sarcoma
- European Commission Grants Orphan Designation to Theriva Biologics’ VCN-01 for Retinoblastoma

