Tonix Pharmaceuticals Submits NDA for TNX-102 SL to Treat Fibromyalgia
Tonix Pharmaceuticals Holding Corp. has announced the submission of a NDA to the U.S. Food and Drug Administration (FDA) for TNX-102 SL (cyclobenzaprine HCl sublingual tablets) 5.6 mg, a non-opioid, centrally-acting analgesic. This drug has shown statistically significant reduction in chronic, widespread pain associated with fibromyalgia in two Phase 3 studies and was generally well tolerated. The FDA granted TNX-102 SL Fast Track designation for fibromyalgia in July 2024, expediting its review process to address this serious condition and unmet medical need.
Publish Date: 16-10-2024 Source: Tonix Pharmaceuticals Holding Corp.
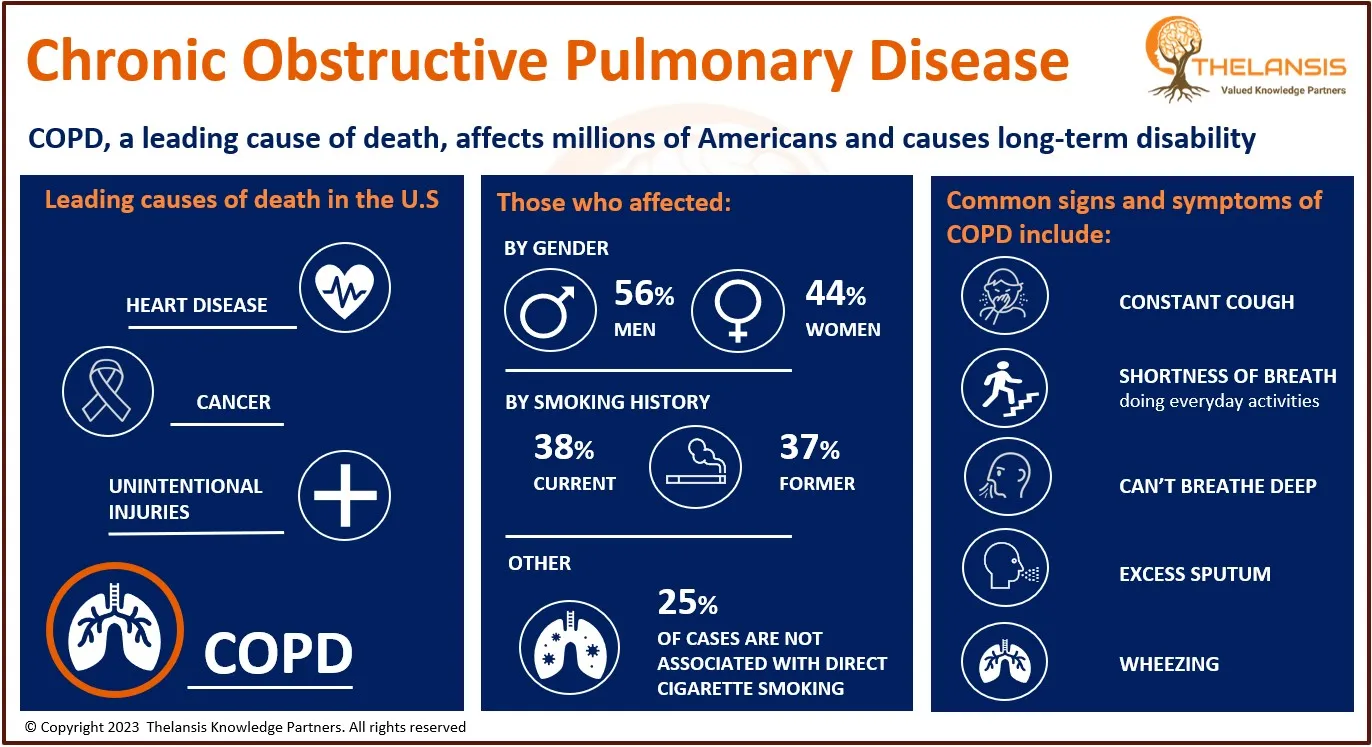
Fibromyalgia (FM) presents a syndrome marked by persistent musculoskeletal pain, known as chronic musculoskeletal pain (CMP). The primary symptoms of FM encompass muscle stiffness, heightened general sensitivity, insomnia, persistent fatigue, mood disorders, cognitive impairment, anxiety, depression, joint stiffness, and an inability to perform everyday tasks. Furthermore, FM can be linked to specific medical conditions, including infections, rheumatic disorders, diabetes, and psychiatric and neurological ailments. FM patients often exhibit a lowered pain threshold, leading to widespread hyperalgesia and allodynia. These phenomena indicate a problem related to pain amplification during sensory processing within the central nervous system (CNS). The diagnosis of FM is generally intricate due to the absence of distinct biochemical, imaging, or pathological characteristics. It is primarily determined through patient history and physical examination, with other diseases being ruled out as part of the diagnostic process. Nevertheless, considering the immune neuroendocrine axis in this syndrome has prompted research into the potential diagnostic and prognostic value of various biomarkers. For the treatment of fibromyalgia, medications like Pregabalin (brand name Lyrica), duloxetine (Cymbalta), and milnacipran HCl (Savella) have received approval from the FDA.
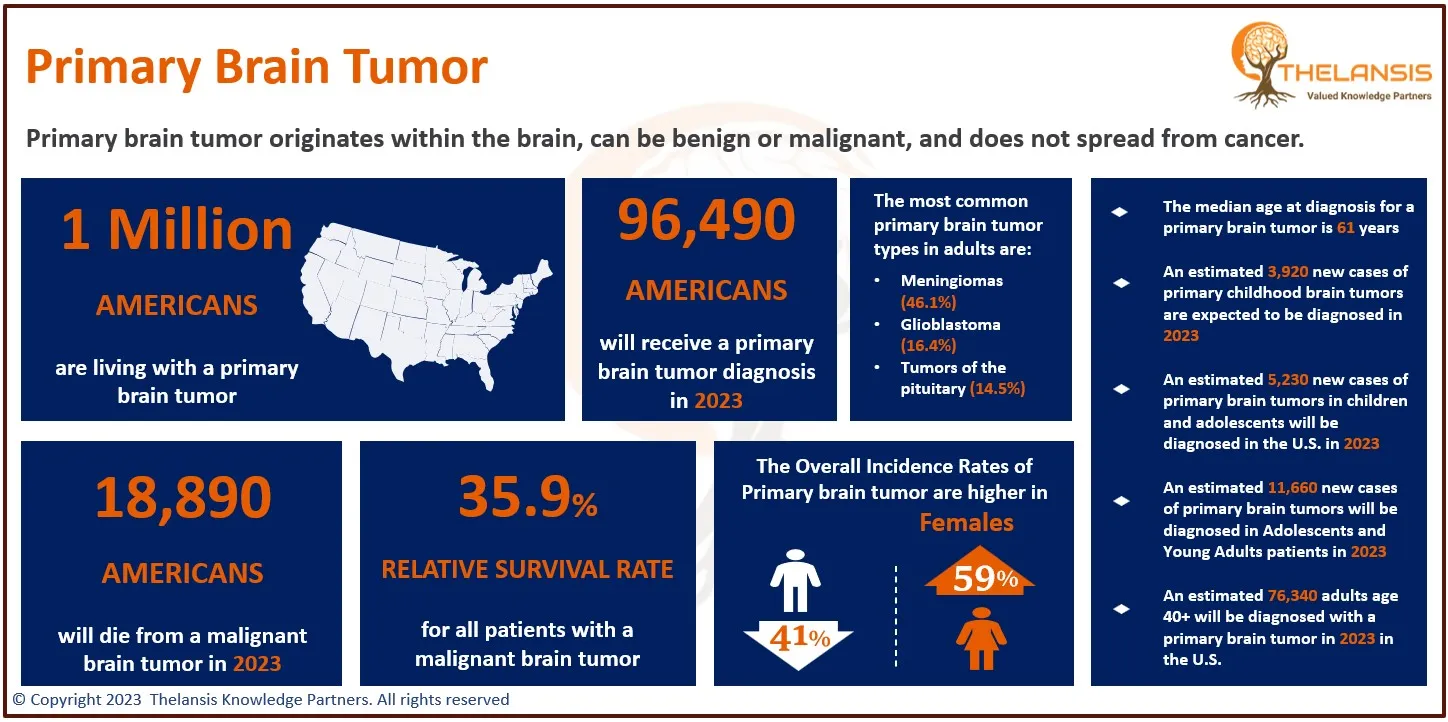
- Fibromyalgia affects approximately 4 million adults in the United States, making up about 2% of the adult population.
However, the current Fibromyalgia treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (Rozanolixizumab, IMC-1, NYX-2925, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Fibromyalgia treatment. The key companies in the advanced development stage are UCB Biopharma SRL, Virios Therapeutics, Inc., Aptinyx, etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Fibromyalgia to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2023 to 2033.
About Thelansis:
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making.
Contact Us:
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com
- Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com