Oct 14 2024
/
FDA Approves Accord BioPharma’s IMULDOSA for Treating Chronic Inflammatory Conditions
Accord BioPharma, Inc., the U.S. specialty division of Intas Pharmaceuticals, Ltd., which focuses on oncology, immunology, and critical care therapies, has announced the FDA approval of IMULDOSA (ustekinumab-srlf). IMULDOSA, a biosimilar to STELARA® (ustekinumab), has been approved for the treatment of various chronic inflammatory conditions, including psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. The FDA has granted approval for all indications that the reference medicine, STELARA, is approved for.
Publish Date: 14-10-2024 Source: Accord BioPharma, Inc.
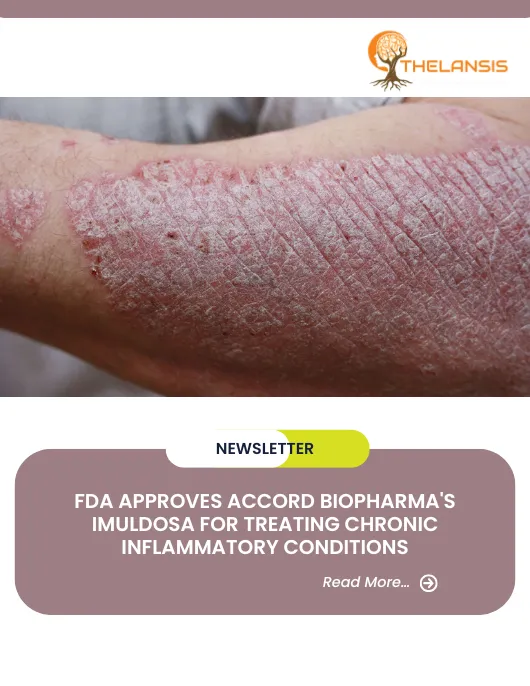
Psoriasis, affecting approximately 2-3% of the global population, is primarily an inflammatory skin condition characterized by abnormal epidermal differentiation and hyperproliferation. The disease’s pathophysiology involves the activation and migration of T-cells to the dermis, leading to the release of cytokines, particularly tumor necrosis factor-alpha (TNF-alpha), which induces inflammation and accelerated skin cell production. This condition manifests with various symptoms, including dry, thick, raised patches on the skin covered by a silvery-white scale. These patches often provoke itching, especially in specific areas like the scalp, lower legs, and groin. In cases where psoriasis affects the hands and feet, painful fissures and cracks may develop, impairing manual tasks and walking. Severe psoriasis on the body can also lead to painful fissures and bleeding. Psoriasis is the most common autoimmune disease attributed to an inappropriate activation of the cellular immune system. The disease’s development hinges on the activation and migration of T cells to the dermal layer, which triggers cytokine release, instigating inflammation and the rapid proliferation of skin cells. The precise trigger for T cell activation remains unknown, but it has long been recognized as a pivotal factor in the disease’s pathogenesis. Numerous clinical studies involving a range of immune-modulating biological agents have been conducted to explore the role of T lymphocytes in psoriasis patients.
- The prevalence of psoriasis varies from 2560 to 3250 cases per 100,000 populations in the USA, with a low diagnosis rate of ~49%.
However, the current Psoriasis treatment market share, market uptake, and attribute analysis concerning the most potential emerging therapies (HB0017, Apremilast, IBI112, Risankizumab, etc..) has been provided under the market outlook section of the study covering 8 MM countries; The United States, EU5 (Germany, Spain, France, Italy, UK) Japan and China.
In terms of pharmacologic therapies, several pharmaceutical products are being approved and under different phases of development for the Psoriasis treatment. The key companies in the advanced development stage are Huabo Biopharm Co., Ltd., Amgen, Innovent Biologics (Suzhou) Co. Ltd., AbbVie, etc..
Based on solid domain and business knowledge, Thelansis Knowledge Partners has published the market outlook forecast report on Psoriasis to provide a clear understanding of disease area background, epidemiology, current and future competitions, the country-specific standard of care, and the complete market forecast for 2023 to 2033.
Thelansis specializes in pharmaceutical market outlook and market forecast reports. We published reports across the therapeutic area, including rare / ultra-rare and mainstream indications. Over the period, we have built a robust repository of 6,000+ Bio-pharma reports that cover Epidemiology studies and Market forecasting based on the KOL opinions.
Competitive intelligence and track of trial results throughout the phases of development executed by a team of a mix of Scientific and Business backgrounds. As an organization, the primary focus is to provide real-world data evidence and market insight to pharmaceutical companies for their decision-making
- Delivery Office:
B-1030, C Wing Vrindavan tech village, Outer ring road
Bangalore- 560037
India+91(124)404-1731
clientsupport@thelansis.com - Sales office:
183 Asylum Street Hartford,
CT-06103, USA
Contact no. +1 (302) 380-3552
m.berg@thelansis.com
Related posts:
- FDA Approves Apellis’ SYFOVRE™ (pegcetacoplan injection) for Geographic Atrophy (GA)
- Stoke Therapeutics Receives FDA Approval to Administer Higher Single Dose of STK-001 in MONARCH Trial for Dravet Syndrome
- Pfizer’s HYMPAVZI Gains FDA Nod for Hemophilia A and B Without Inhibitors
- FDA Approves AbbVie’s VYALEV for 24-Hour Treatment of Advanced Parkinson’s Disease

