
FDA Grants Orphan Drug Designation to Ezurpimtrostat (GNS561) for Hepatocellular Carcinoma (HCC) Treatment
Genoscience Pharma today announces that its lead candidate, ezurpimtrostat (GNS561), a PPT-1 (Palmitoyl Protein Thioesterase-1) inhibitor, has been granted Orphan Drug Designation by the U.S. FDA for ...
4D Molecular Therapeutics Receives FDA Clearance for IND Application of 4D-150 for Diabetic Macular Edema Treatment
4D Molecular Therapeutics, a clinical-stage biotherapeutics company using directed evolution to create targeted genetic medicines, announced that the Food and Drug Administration (FDA) has approved th ...
Anavex Announces Exceeding of Enrollment Target for the ANAVEX®2-73 (blarcamesine) EXCELLENCE Phase 2/3 Rett Syndrome Clinical Trial
Anavex Life Sciences Corp., a clinical-stage biopharmaceutical company, announced that 92 patients with Rett syndrome have been enrolled in the ANAVEX®2-73 (blarcamesine) EXCELLENCE Phase 2/3 study in ...
FDA Approves TEZSPIRE® for Self-Administration in Severe Asthma Patients Aged 12+
Amgen and AstraZeneca announced today that TEZSPIRE® (tezepelumab-ekko) has received approval from the U.S. FDA for self-administration in a pre-filled, single-use pen for severe asthma patients aged ...

FDA Grants Orphan-Drug Exclusivity to Recorlev® for Endogenous Cushing’s Syndrome Treatment
Xeris Pharmaceuticals has revealed that the FDA has awarded orphan-drug exclusivity for its drug Recorlev® (levoketoconazole) for treating adult patients with endogenous Cushing's syndrome for whom su ...
XORTX Therapeutics Submits Orphan Drug Designation Request for XRx-008 Program to FDA for ADPKD Treatment
XORTX Therapeutics Inc., a late-stage clinical pharma company focused on developing novel therapies to treat progressive kidney disease, has submitted an Orphan Drug Designation request to the U.S. Fo ...

FDA Grants Orphan Drug Status to Beactica’s BEA-17 for Glioblastoma Treatment
Beactica Therapeutics, a Swedish precision oncology company, announced today that the U.S. FDA has designated BEA-17 as an orphan drug for the treatment of glioblastoma (GBM). BEA-17 is a first-of-its ...

FDA Grants Fast Track Designation for Reneo Pharma’s Mavodelpar (REN001) for LCHAD Deficiency Treatment
Reneo Pharmaceuticals, Inc., a clinical-stage pharmaceutical company focused on developing and commercialising therapies for patients with rare genetic mitochondrial diseases granted Fast Track design ...
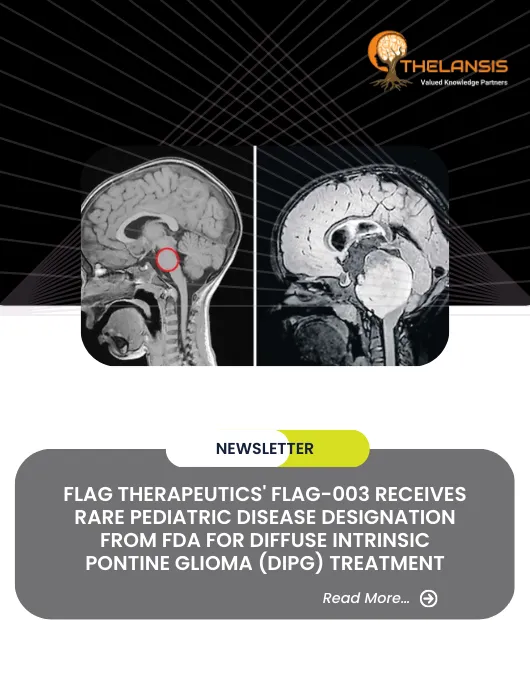
FLAG Therapeutics’ FLAG-003 Receives Rare Pediatric Disease Designation from FDA for Diffuse Intrinsic Pontine Glioma (DIPG) Treatment
FLAG Therapeutics announces FLAG-003 granted Rare Pediatric Disease designation by FDA. FLAG-003 is an investigational therapy for treating Diffuse Intrinsic Pontine Glioma (DIPG), a rare, aggressive ...

Moderna’s mRNA-1345 Receives Breakthrough Designation from FDA for Respiratory Syncytial Virus (RSV) Prevention
Moderna, a biotechnology company pioneering mRNA therapeutics and vaccines, announced that mRNA-1345, its investigational mRNA vaccine for respiratory syncytial virus (RSV), has received Breakthrough ...

