Feb 10 2026
/
Designing an Early Access Pathway for a CNS Therapy Using Real-World and Claims Insights
Background:
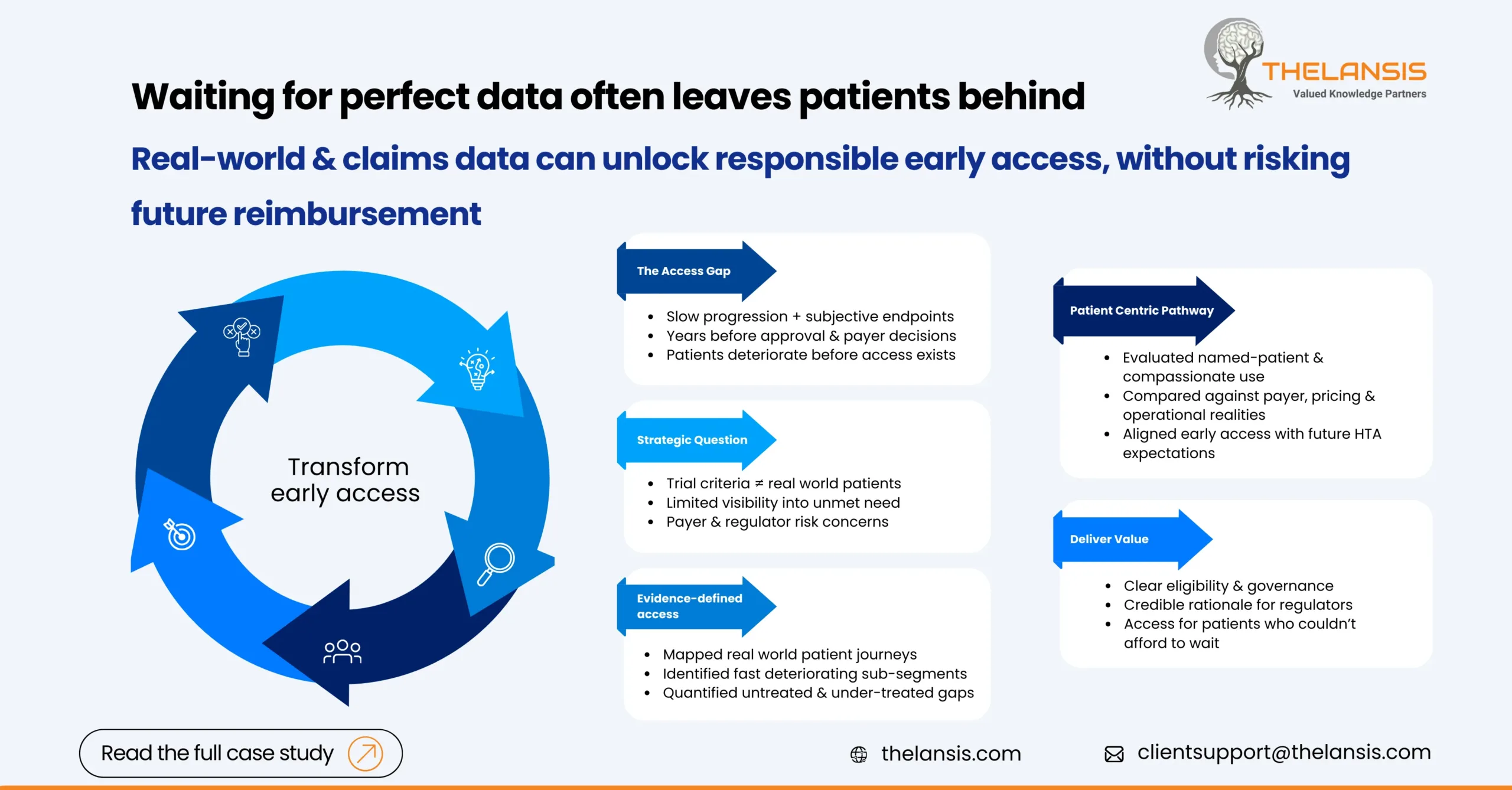
A mid-stage biopharma company was advancing a novel CNS therapy into late Phase II, with early clinical signals suggesting meaningful benefit for patients with progressive neurological decline. While the science was promising, leadership faced a familiar CNS challenge: patients needed treatment sooner than regulatory and reimbursement timelines would allow.
Slow disease progression, subjective clinical endpoints, and long follow-up periods meant approval and payer decisions were still years away. Meanwhile, clinicians were encountering patients with limited alternatives, highlighting a growing access gap well before launch.
The Challenge:
The company needed an early access strategy that balanced urgency with responsibility. Key challenges included:
- Defining which real-world patients should qualify beyond the trial population
- Limited visibility into current treatment pathways and unmet needs
- Payer and regulator concerns around uncontrolled access and future reimbursement risk
- CNS-specific complexity, where short-term outcomes are difficult to measure
Objective:
The client partnered with Thelansis to design an asset-specific early access pathway that would:
- Enable responsible pre-approval patient access
- Use real-world and claims data to ground decisions in evidence
- Align early access design with future payer and HTA expectations
- Support regulatory, ethics committee, and clinician discussions with defensible insights
Our Approach:
1. Mapping the Real-World Patient Landscape
Using longitudinal claims and real-world datasets, we reconstructed how patients were actually diagnosed, treated, and progressed outside clinical trials. This allowed us to:
- Identify patient sub-segments most likely to deteriorate before approval
- Quantify delays between diagnosis, treatment initiation, and disease progression
- Highlight points where patients routinely fell outside standard care pathways
This step reframed early access from a “compassionate option” into a data-defined intervention for a specific unmet need.
2. Quantifying Unmet Need and Treatment Gaps
We translated real-world patterns into clear metrics that resonated with decision-makers:
- Size of the population with no viable treatment alternatives
- Duration of untreated or sub-optimally treated disease
- Geographic and system-level variation in access
These insights formed the backbone of the early access rationale, helping move discussions beyond anecdote toward evidence.
3. Designing an Evidence-Backed Early Access Model
Based on market dynamics and regulatory norms, we evaluated multiple pathways, including:
- Named-patient programs
- Managed access agreements
- Compassionate use frameworks
Each option was stress-tested against payer expectations, future pricing considerations, and operational feasibility. The result was a targeted early access design, not a one-size-fits-all program.
4. Supporting Stakeholder Dialogue
We translated complex data into clear, credible narratives tailored for different audiences:
- Regulators and ethics committees: patient safety, eligibility logic, and governance
- Payers: population size control, evidence generation, and downstream value
- Clinicians: clarity on who qualifies and why
Importantly, early access was positioned as a bridge to launch, not a substitute for reimbursement.
Outcome:
The client emerged with a clearly defined early access pathway that:
- Enabled pre-approval access for precisely defined patient segments
- Provided regulators and ethics committees with a credible, evidence-based rationale
- Preserved long-term pricing and reimbursement integrity
- Supported patients who could not afford to wait for full approval
In CNS, waiting for perfect data often leaves patients behind. This case demonstrates how real-world and claims insights can transform early access from a reactive measure into a strategic, patient-centric pathway.

