Feb 13 2026
/
The Hidden Gap Between Phase III Success and Market Access: When Evidence Misalignment Delays Commercial Readiness
For many biopharma teams, Phase III is treated as the final mountain to climb. Once efficacy and safety are proven, commercial readiness is assumed to follow naturally. In reality, that assumption is one of the most costly missteps in drug development.
More assets than ever are reaching regulatory approval on time, yet facing slower-than-expected uptake, prolonged access negotiations, or restrictive reimbursement decisions. The issue isn’t clinical failure. It’s evidence of misalignment.
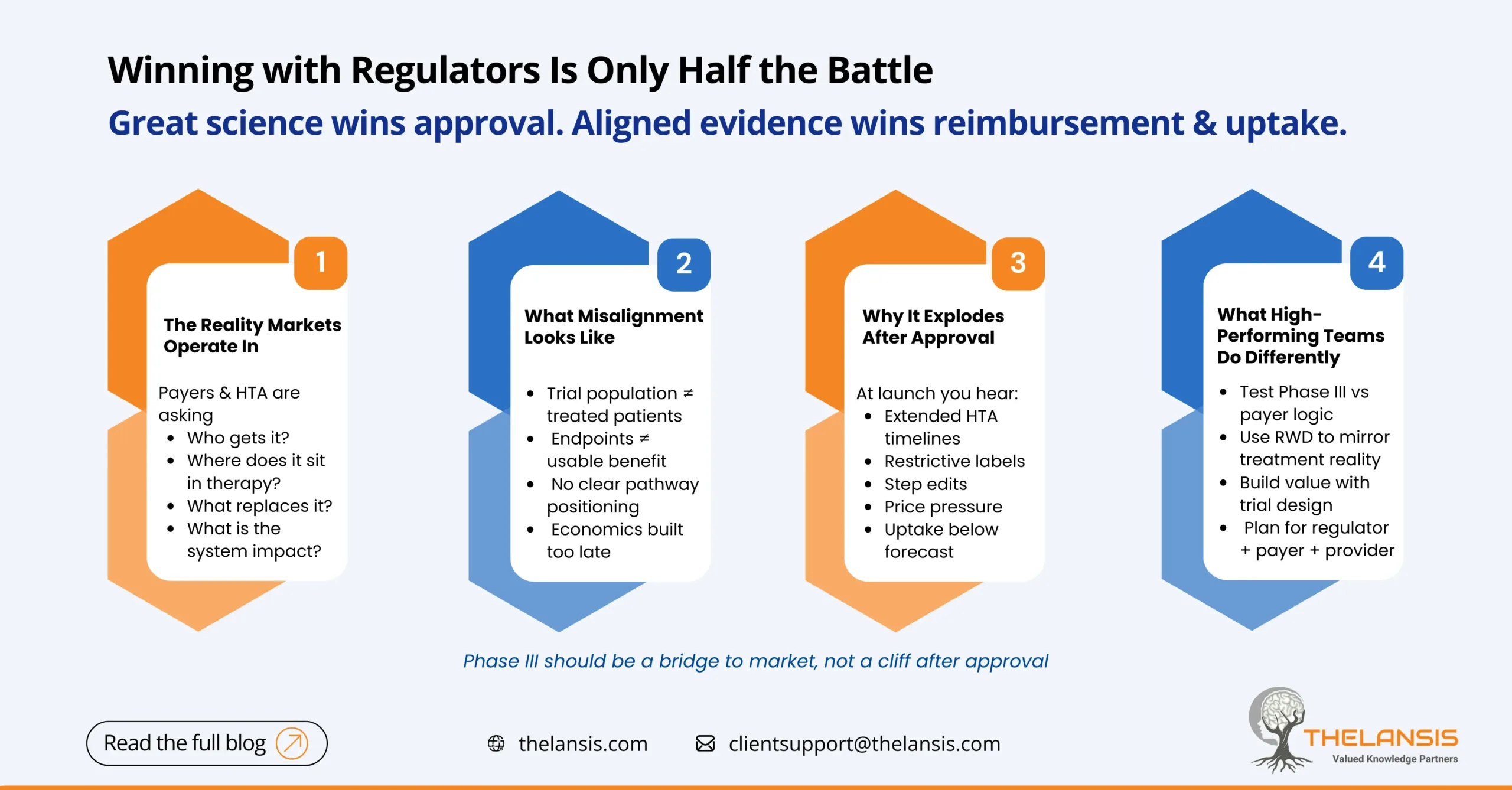
Approval is Not the Same as Readiness
Phase III trials are designed to answer a specific question: Does the therapy work, and is it safe? Regulators care deeply about this, and rightly so. But payers, HTA bodies, and health systems are solving a different problem altogether. They are deciding who should receive the therapy, when, and at what cost to the system, rather than what.
When evidence is optimized only for approval, commercial teams are often left trying to retrofit value stories after the design choices are already locked.
What Evidence Misalignment Looks Like in Practice
Misalignment doesn’t mean missing data. It usually means the right data exists, but not in a form that decision-makers can use.
Common signs include trial populations that don’t resemble treated patients, endpoints that are hard to translate into real-world benefit, unclear positioning within treatment pathways, and economic models built too late to shape strategy. Individually, these gaps seem manageable. Together, they slow access.
Phase III Answers One Question, Markets Ask Several
Beyond clinical performance, markets want to understand comparative value, durability of benefit, real-world applicability, budget impact, and behavioral change in clinical practice.
Markets don’t start questioning after approval, but many teams wait until then to engage with them seriously.
The result is often prolonged HTA reviews, conservative pricing outcomes, step edits, or cautious adoption that lags far behind launch forecasts.
Why the Delay is Often Invisible Until Launch
Evidence misalignment rarely shows up as a red flag during development. It surfaces later as “unexpected” payer resistance or uneven uptake across markets. By that stage, timelines are compressed, pricing flexibility is limited, and additional studies become reactive rather than strategic.
What looks like a launch problem is usually a much earlier evidence planning issue.
The Shift High-Performing Teams Are Making
The fix isn’t more data, it’s earlier alignment. Leading teams are pressure-testing Phase III evidence against payer logic well before readout, using real-world and claims data to understand treatment reality, and shaping value narratives alongside clinical results rather than after them.
Commercial readiness today means evidence that works across regulators, payers, and providers — not just one audience.
Final Thought
Phase III should act as a bridge to the market, not a cliff edge. When evidence is aligned early with how access decisions are actually made, strong science translates faster into real-world impact. When it isn’t, even successful trials can quietly delay the very patients they were meant to help.

