Nov 11 2025
/
EU Pharmaceutical Supply Chain: How to Shrink Upstream Risk Without Shrinking Access
Europe’s pharmaceutical landscape is undergoing a significant change due to its medicine shortage problem, which has a stubborn root cause: fragile upstream links. The situation becomes apparent at the pharmacy counter, but it originates much earlier, at the level of raw materials, intermediates, and APIs, which are overwhelmingly sourced outside the EU. Recent audits and policy moves acknowledge this head-on; the European Court of Auditors (ECA) pointed out chronic shortages through 2024, driven by dependence on Asian suppliers and thin market incentives, as more than 80% of antibiotic APIs used in Europe are imported from Asia.
Over the last 18 months, Brussels has moved from diagnosis to action. There’s now a Union List of Critical Medicines updated in December 2024, and HERA has published a vulnerability assessment for that list. In March 2025, the Commission proposed the Critical Medicines Act with tools such as strategic projects, stockpiling, and supply chain transparency. Member States have also co-funded manufacturing upgrades for hard-to-move antibiotics capacity.
This piece looks at practical strategies EU buyers, regulators, and manufacturers can use to cut upstream vulnerability on the basis of what’s already happening on the ground.
Where is the upstream most brittle?
Concentrated API and intermediate supply: For many critical molecules, a handful of sites in China or India produce a large share of global output. When one plant goes down, ripple effects hit Europe months later. HERA’s 2024 pilot mapped these structural single-points-of-failure across selected critical medicines.
Procurement that rewards the cheapest single source: For generics, awarding tenders primarily on price has hollowed out redundant capacity in Europe. The Commission is urging multi-criteria tenders to avoid single-winner, race-to-the-bottom dynamics.
Cost and scale headwinds for reshoring: Independent analyses argue Europe can match Asia on quality and process efficiency, but capital and operating costs and fragmented demand would still bite. That’s why targeted state aid has focused on strategic bottlenecks like β-lactam antibiotics.
What’s changed in 2024–2025
A codified risk lens: The EMA’s Union List (Dec 2024) formalized which medicines merit priority attention, providing a basis for coordinated interventions.
Systematic vulnerability mapping: HERA’s technical report (July 2024) catalogs risks along the chain and companies can use this data when prioritizing audits and dual sourcing.
A policy toolkit: The proposed Critical Medicines Act would enable strategic projects, mandatory transparency, and stockpiling obligations, shifting resilience from ad-hoc to governed.
Real manufacturing moves: Sandoz expanded its Austria site in Kundl, Europe’s last end-to-end penicillin hub supported by EU-Austria measures, while France backed a new paracetamol API facility under Seqens at the Roussillon platform.
Regulation and logistics: new levers for resilience
At the regulatory level, the EU is advancing its broader pharmaceuticals reform, known as the “Pharma Package”, which was endorsed by the Council of the European Union on 4 June 2025. This reform updates fundamental rules about obligations to supply, tighter oversight of shortages, and refreshed incentives to develop onshore manufacturing and distribution capability. Combined, these shifts suggest a more assertive posture by regulators in moving from disaster management to resilience planning.
On the logistics front, Europe’s cold-chain services market is projected to grow about USD 21.5 billion in 2025. The surge is driven by a rising share of biologics, cell, and gene therapies that demand ultra-low temperature storage and transport. Notably, Central and Eastern Europe are expected to register some of the fastest growth rates in pharmaceutical cold-chain logistics over the next few years.
Operationally, this reshapes the map for pharmaceutical manufacturers, contract partners, and logistics providers. Eastern European distribution hubs can no longer be viewed as low-cost back offices; they are emerging as strategic nodes in maintaining continuity across EU supply networks. Because supply flows are concentrated through a limited number of key trade corridors, flexibility is thin and the stakes are high.
As medicine shortages increasingly stem from logistical breakdowns rather than manufacturing shortfalls, companies are now expected to stress-test cold-chain integrity, transit times, and cross-border regulatory compliance as part of standard planning. But data fragmentation across temperature tracking, transport milestones, and customs checkpoints remains a major barrier to real-time visibility and rapid response. While new policies and investments mark genuine progress toward resilience, the logistics backbone of the EU pharmaceutical chain remains vulnerable.
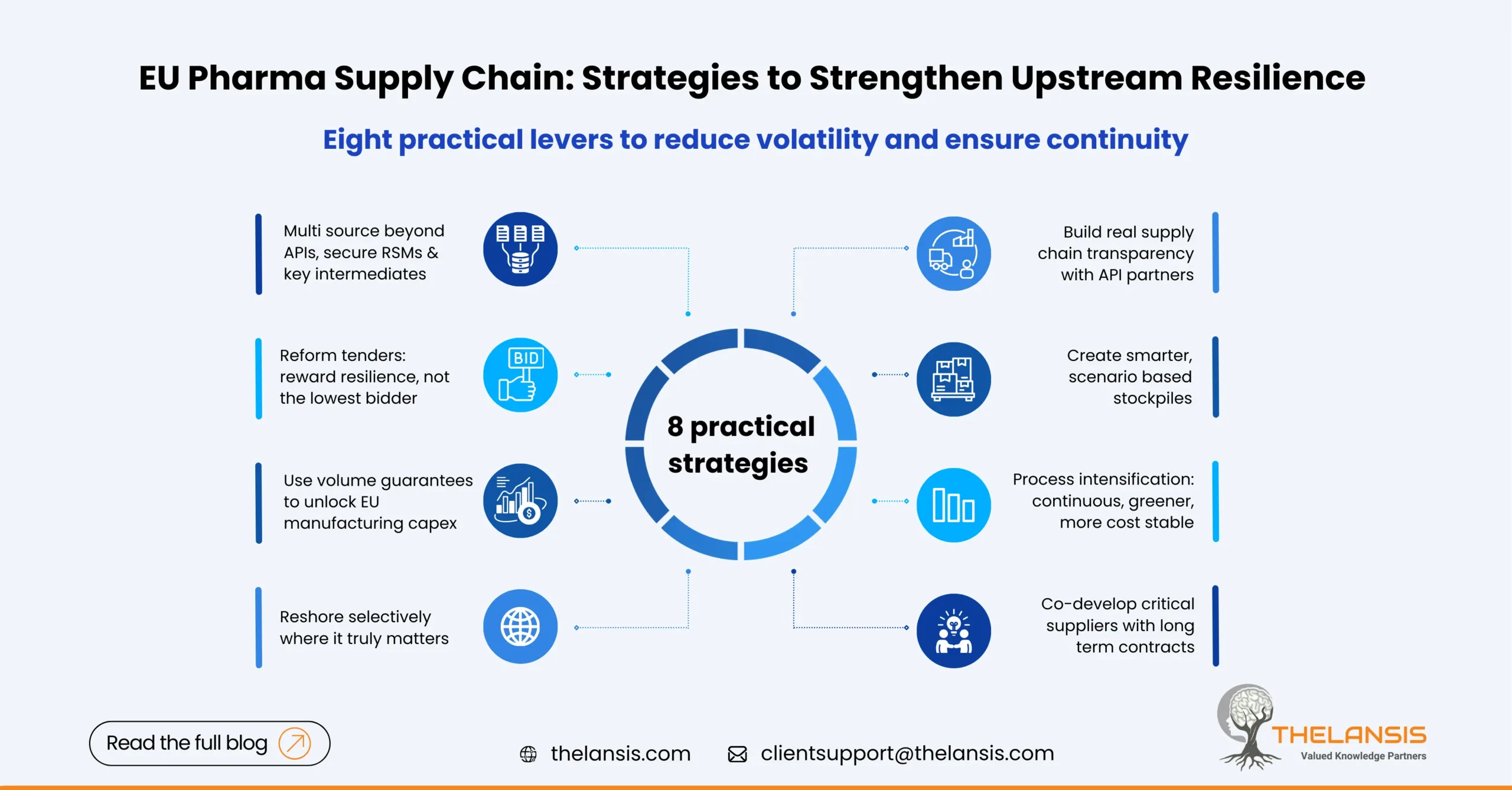
Eight strategies that actually reduce upstream vulnerability
1. Dual- and multi-source the right links and not just the API
Risk rarely starts at the final API; it often begins with regulated starting materials (RSMs) and key intermediates. Build a “qualified second source” program that includes alternate suppliers for at least one RSM and the main intermediate, plus the API. Prioritize molecules on the Union List first, using HERA’s mapping to pick your battles.
2. Change how EU buyers purchase generics
Move away from single winner, price only tenders toward multi-winner frameworks with resilience criteria (e.g., geographically diversified API sources, minimum buffer stocks, and proof of alternate sites). The Commission has explicitly urged contracting authorities to consider non-price criteria for critical molecules; mirroring that in national tender rules would keep a second supplier viable.
3. Use volume guarantees to unlock capex
European plants struggle to justify investments if demand is fragmented or short-term. Long-horizon volume commitments and take-or-pay contracts anchored by hospital groups and payers can underwrite new assets. Austria’s support for Kundl and the EU “strategic project” designation under the Critical Medicines Act provides templates to bundle public co-funding with private capex.
4. Targeted reshoring and “friend-shoring”
Not everything must be made in the EU, but a subset should.
Criteria: (a) few global producers, (b) frequent shortages, (c) high clinical criticality, (d) feasible process intensification. The Kundl expansion for penicillin and the French paracetamol project illustrate where public money plus process upgrades can shift the risk calculus.
5. Build data transparency beyond regulatory minimums
Even robust QP and serialization frameworks don’t reveal real-time capacity or inventory at suppliers. The proposed Act points to enhanced supply chain transparency and regular reporting. Companies can get ahead by creating secure “data rooms” with tier-1 API suppliers that include lead times for key solvents, energy-cost indexation, and planned shutdowns, enabling dynamic allocation before a shortage hits.
6. Smarter stockpiles, not just bigger ones
Inventories should be scenario-based, for example, 8–12 weeks for antibiotics, where Asia supplies >80% of API, and rotated through hospital demand to avoid wastage. EU-level stockpiling is useful, but auditors warn that blunt stockpiles can distort markets if they starve other regions; coordination and transparency are essential.
7. Process intensification and greener routes to cut OPEX risk
Continuous processing, better catalysts, and solvent recovery reduce exposure to energy spikes and waste costs, these are the key reasons Europe isn’t cost-competitive today. Industry analyses show Europe can match Asia when processes are modern and integrated, resilience and sustainability goals align here.
8. Segment the supplier base and co-develop
For a handful of critical APIs, run supplier development programs: audit readiness, alternate route validation, and CEP/ASMF document support to accelerate second-source approvals. Tie this to long-term contracts, not single-tender cycles, so suppliers have a business case to keep capacity warm.
Bottom line
Europe won’t and doesn’t need to onshore everything. But it does need to de-risk the fragile upstream nodes for a defined list of critical medicines. The tools are finally on the table: better tenders, targeted capex, structured transparency, and coordinated stockpiles. The examples at Kundl and Roussillon show the model: pick the right molecules, underwrite capacity with real offtake, and modernize processes so European plants become competitive. If 2024 was about mapping the problem, 2025 should be about locking in redundant upstream capacity where it counts most.

