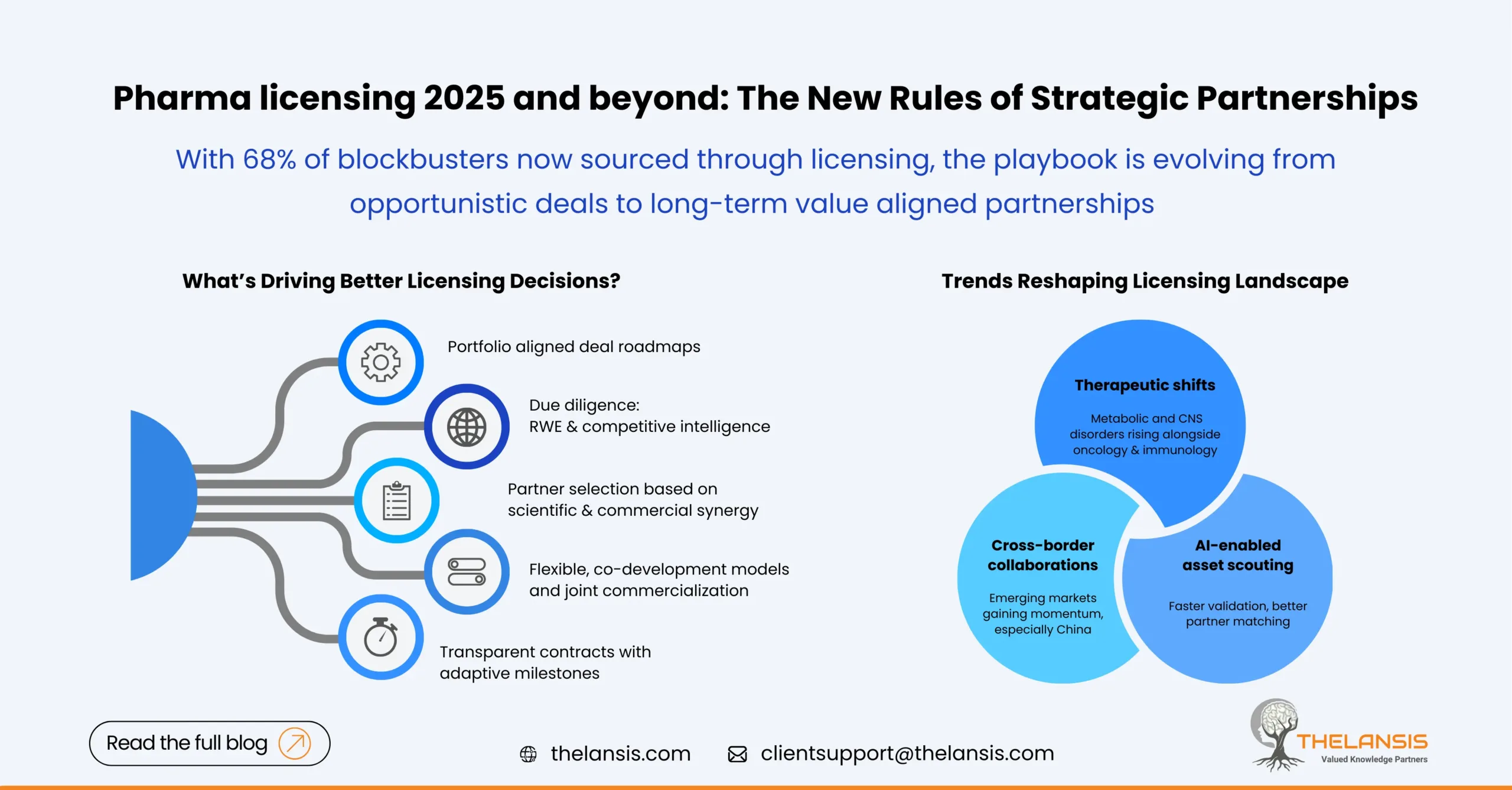
Pharma Licensing 2025 and Beyond: Rethinking Strategic Partnerships for Long-Term Value
Introduction: Licensing as a Growth Engine in Pharma The global pharmaceutical and biotech companies are entering an era of collaboration, agility, and strategic alliances. As the R&D cost rises ...
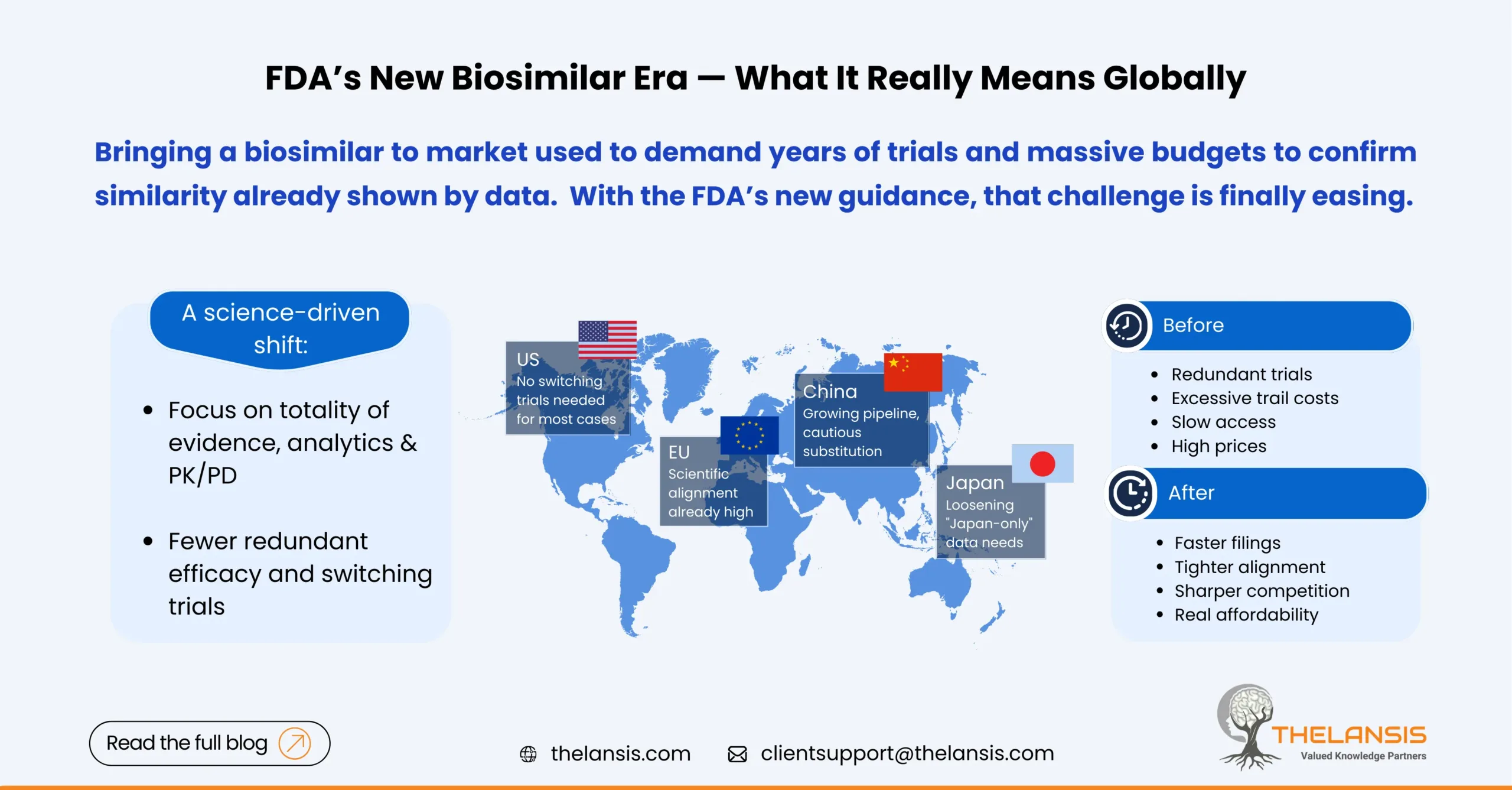
FDA’s Updated Biosimilar Policy: What it really means in the US, EU5, Japan, and China
The regulatory landscape for biosimilars is shifting significantly in 2025. The FDA has taken two big steps that collectively make it faster and cheaper to bring many biosimilars to market:In June 202 ...
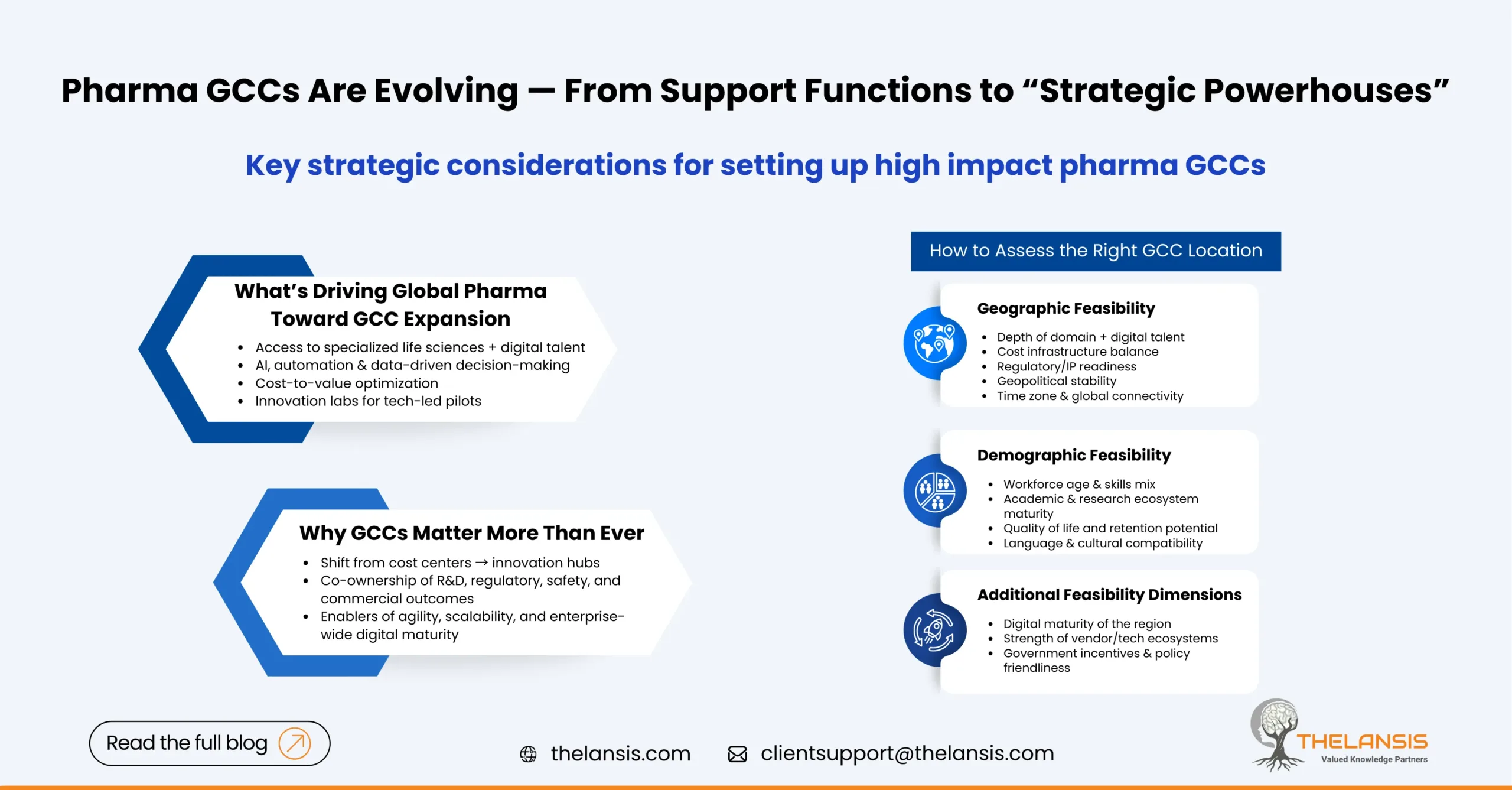
Pharma GCC Setup: A Strategic Feasibility Framework for Location and Talent Decisions
The pharma industry has seen a wave of change over the decades, driven by digital innovation, data analytics & global collaboration. A major catalyst behind this change has been the rise of Global ...
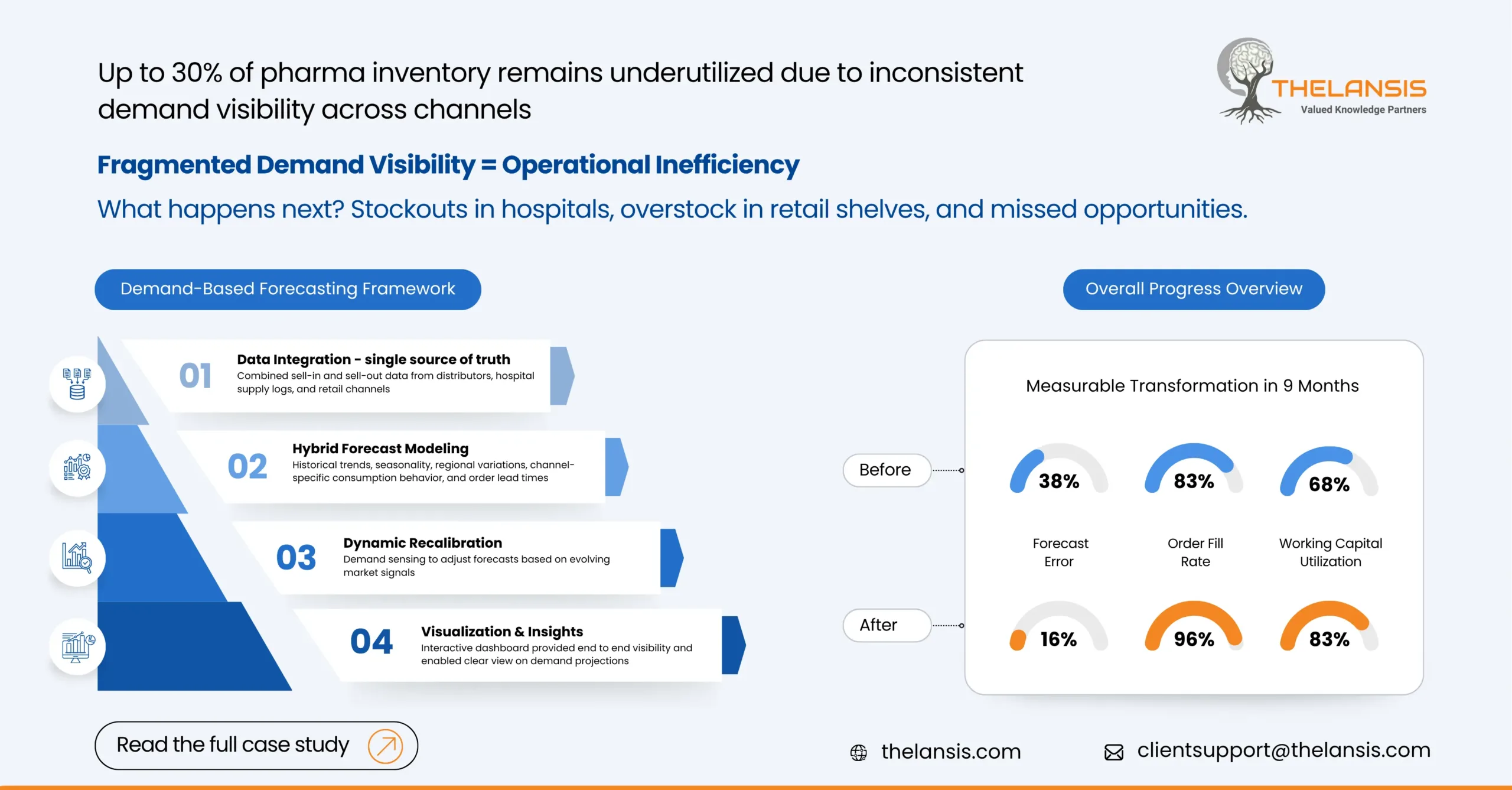
Optimizing Multi-channel Distribution and Inventory Agility in Pharmaceutical Industry through Demand-Based Forecasting
Introduction: The pharma ecosystem is quite complex, wherein companies operate across various channels, ranging from hospital procurement networks to retail pharmacies. While this expands their marke ...
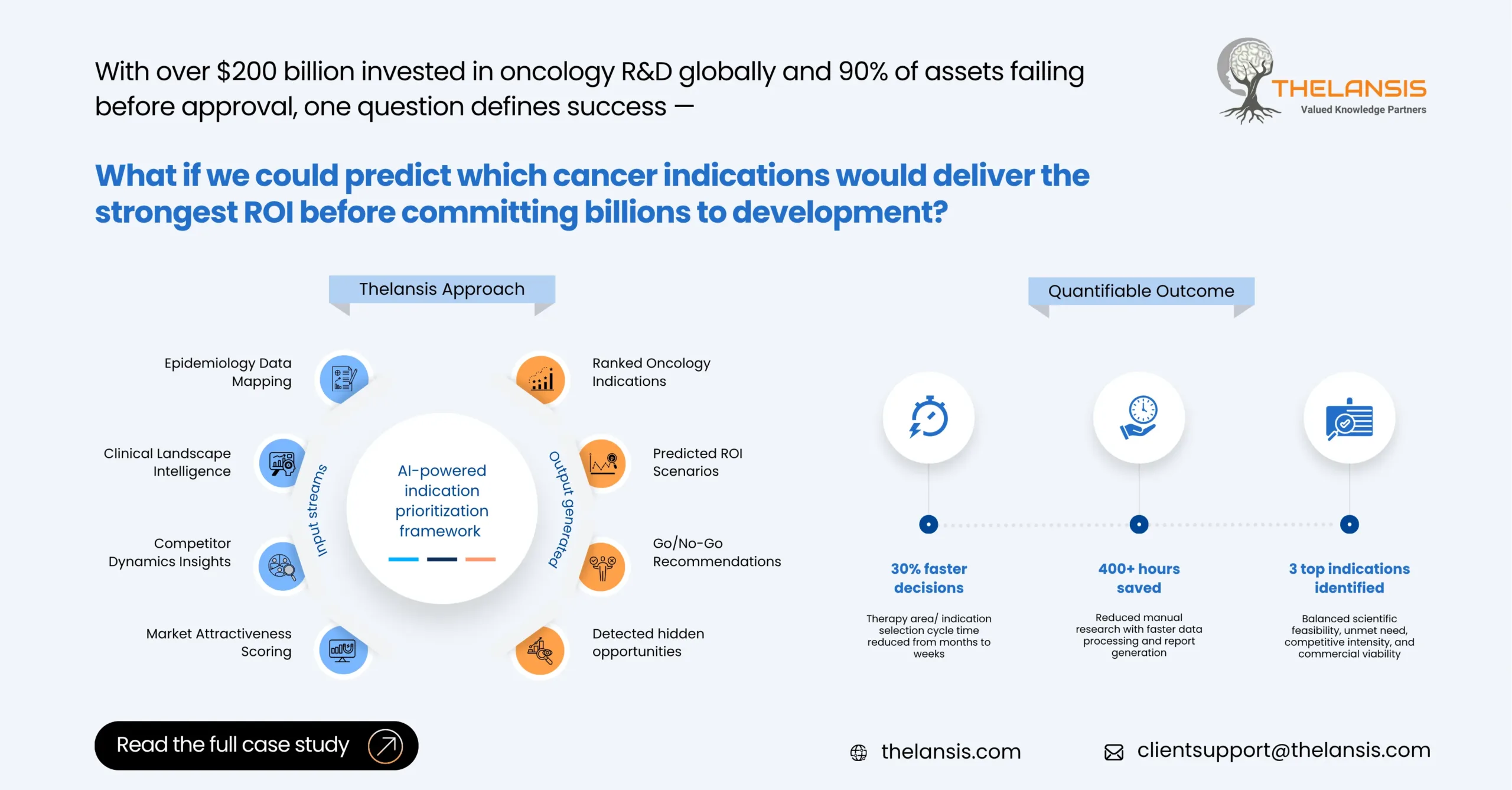
Smart Pipeline Decisions: AI-Powered Indication Prioritization for Oncology TA
Background: In the current scenario, the competitive landscape of oncology TA, where innovation moves rapidly, pharma companies face intense pressure to optimize their pipeline investments. With hund ...

Mapping the Future: Identifying High-value Therapy Area Strategy for Emerging Markets
Background: In an evolving global disease landscape, identifying the right therapy areas or indication setting for investment is a strategic move that defines a company's growth trajectory, especiall ...

The Analogue Advantage: A Smarter Way to derive the market uptake and New Product revenue Forecast
Background: Launching a new therapy in the oncology space is one of the most complex and challenging in the pharma and biotech industries. With evolving treatment landscapes, shifting market dynamics ...

From Evidence to Impact: How Thelansis Accelerated a Rare Disease Submission
Introduction: In recent years, the healthcare landscape has evolved toward an evidence-based, patient-centric architecture. Although conventional clinical trials remain the gold standard, they do not ...

Anticipating the Next Move: AI at the Core of Competitive Intelligence
Introduction: In today's competitive pharmaceutical industry, success relies on anticipating market changes. With rapidly growing data sources, AI-powered data analytics solutions, and predictive mod ...

Next-Gen Data Access for Pharma: How Integrated Data Ecosystems Are Powering Global Pharma Decision-Making
Introduction: In today’s dynamic pharmaceutical landscape, the need for seamless data access and faster decision-making has made AI-powered, cloud-based solutions a cornerstone of innovation. As a ph ...

