Nanoscope Therapeutics Prepares BLA Submission for MCO-010 in Retinitis Pigmentosa Following FDA Meeting
Nanoscope Therapeutics Inc., a late-stage clinical biotechnology company dedicated to developing gene therapies for retinal degenerative diseases, has announced a successful FDA meeting for its clinic ...
FDA Grants Rare Pediatric Disease Designation to Papillon Therapeutics’ PPL-001 for Friedreich’s Ataxia
Papillon Therapeutics Inc., a clinical-stage biotechnology company focused on developing multi-systemic genetic medicines to address the root causes of inherited diseases, has announced that the U.S. ...
FDA Grants Orphan Drug Designation to Immuneering’s IMM-1-104 for Pancreatic Cancer Treatment
Immuneering Corporation, a clinical-stage oncology company focused on developing universal RAS/RAF medicines for a broad range of cancer patients, has announced that the U.S. Food and Drug Administrat ...
Pfizer’s HYMPAVZI Gains FDA Nod for Hemophilia A and B Without Inhibitors
Pfizer Inc. announced the FDA approval of HYMPAVZI™ (marstacimab-hncq) for routine prophylaxis aimed at preventing or reducing the frequency of bleeding episodes. This approval covers both adult and p ...
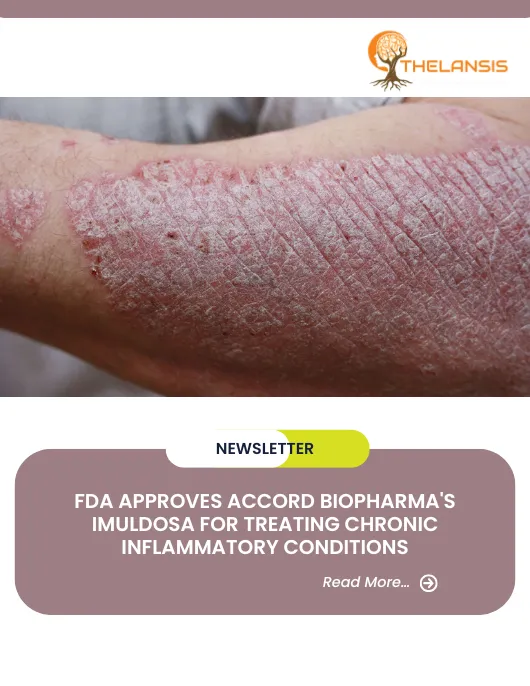
FDA Approves Accord BioPharma’s IMULDOSA for Treating Chronic Inflammatory Conditions
Accord BioPharma, Inc., the U.S. specialty division of Intas Pharmaceuticals, Ltd., which focuses on oncology, immunology, and critical care therapies, has announced the FDA approval of IMULDOSA (uste ...
Capricor Therapeutics Begins BLA Submission for Deramiocel to Treat DMD Cardiomyopathy
Capricor Therapeutics, a biotechnology company dedicated to developing transformative cell and exosome-based therapeutics for rare diseases, has announced the initiation of its rolling submission proc ...
Dizal’s Sunvozertinib Receives Breakthrough Therapy Designation for First-Line NSCLC Treatment
Dizal, a biopharmaceutical company dedicated to the development of novel treatments for cancer and immunological diseases, has announced that the Center for Drug Evaluation (CDE) of China's National M ...

Clearmind Medicine Receives IRB Approval for Phase I/IIa Clinical Trial of CMND-100 Targeting Alcohol Use Disorder
Clearmind Medicine Inc., a clinical-stage biotech company dedicated to the discovery and development of innovative psychedelic-derived therapeutics for major under-treated health issues, has received ...

FDA Grants Breakthrough Therapy Designation to Mirum’s Volixibat for Cholestatic Pruritus in PBC
Mirum Pharmaceuticals, Inc. has announced that the U.S. Food and Drug Administration (FDA) has awarded Breakthrough therapy designation to their drug, volixibat, as a potential treatment for cholestat ...

2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 2)
[vc_row css=".vc_custom_1715260973671{margin-top: -30px !important;}"][vc_column][vc_custom_heading text="2024 ISPOR Annual Meeting Highlights – Anticipated impact of Inflation Reduction Act (Part 2)" ...
