FDA Extends PDUFA Date for Neurotech’s NT-501 Eye Cell Therapy Implant
FDA Extends PDUFA Date for Neurotech’s NT-501 Eye Cell Therapy Implant Neurotech Pharmaceuticals' eye cell therapy implant has faced a delay, as the US Food and Drug Administration (FDA) extended the ...

FDA Clears CytoDyn’s Leronlimab for Phase II Trial in Colorectal Cancer
FDA Clears CytoDyn’s Leronlimab for Phase II Trial in Colorectal Cancer CytoDyn Inc., a biotechnology company specializing in the development of leronlimab, a CCR5 antagonist with potential for multip ...
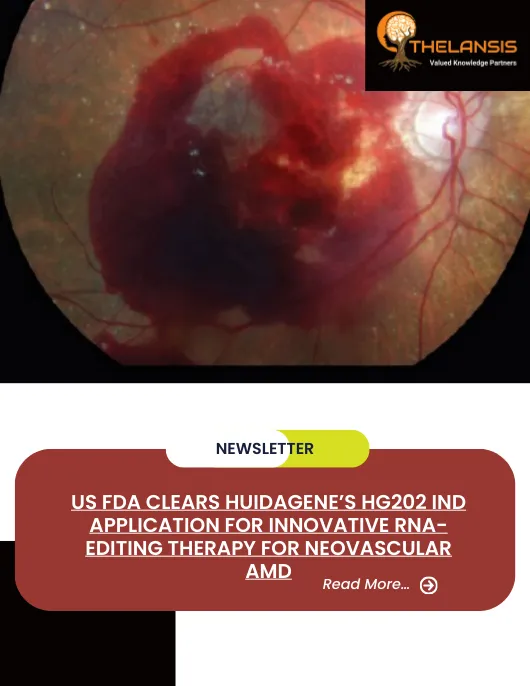
US FDA Clears HuidaGene’s HG202 IND Application for Innovative RNA-Editing Therapy for Neovascular AMD
HuidaGene Therapeutics (HuidaGene), a global clinical-stage biotechnology company specializing in genome medicines, has announced that the US FDA has cleared its HG202 investigational new drug (IND) a ...
FDA Fast Tracks FELIQS’ FLQ-101 for Preventing Retinopathy of Prematurity (ROP)
The U.S. Food and Drug Administration (FDA) has granted fast-track designation to FELIQS' lead product, FLQ-101, which aims to prevent retinopathy of prematurity (ROP). FLQ-101 can be administered ora ...
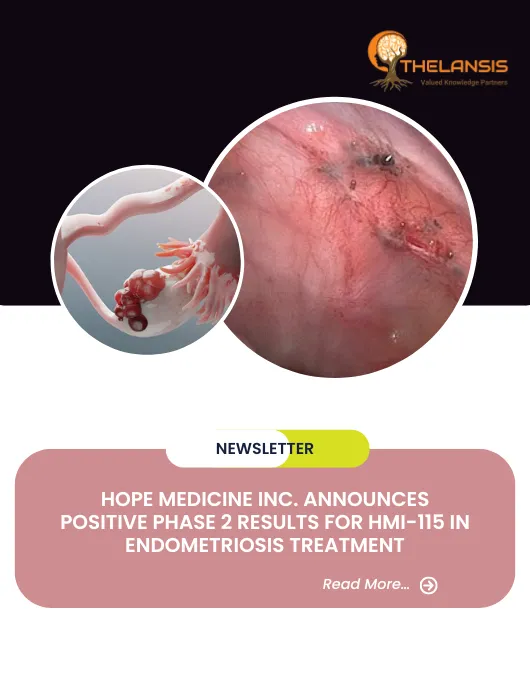
Hope Medicine Inc. Announces Positive Phase 2 Results for HMI-115 in Endometriosis Treatment
Hope Medicine Inc., a clinical-stage innovative biopharmaceutical company, announced positive results from an interim analysis of a global Phase 2 study, titled "A Randomized, Multicenter, Double-Blin ...
FDA Grants Orphan Drug Designation to Kind Pharmaceutical’s AND017 for Sickle Cell Disease
Kind Pharmaceutical, a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, announced that the U.S. Food and Drug Administra ...
FDA Grants Fast Track Designation to HiberCell’s HC-7366 for Acute Myeloid Leukemia Treatment
HiberCell, Inc., a clinical-stage biotechnology company focused on developing therapeutics to combat advanced cancer and cancer resistance, announced that the U.S. Food and Drug Administration (FDA) h ...

FDA Clears Autobahn’s IND for Phase 2 Trial of ABX-002 in Bipolar Depression
Autobahn Therapeutics, a biotech company focused on developing restorative treatments for neuropsychiatric and neuroimmunologic disorders, announced the clearance of an investigational new drug (IND) ...
FDA Approves AbbVie’s VYALEV for 24-Hour Treatment of Advanced Parkinson’s Disease
AbbVie announced that the U.S. FDA has approved VYALEV™ (foscarbidopa and foslevodopa) as the first and only subcutaneous 24-hour infusion of levodopa-based therapy for treating motor fluctuations in ...
Sangamo Therapeutics Gains FDA Pathway to Accelerated Approval for ST-920 Gene Therapy in Fabry Disease
Sangamo Therapeutics, Inc., a genomic medicine company, announced the outcome of a recent successful interaction with the U.S. Food and Drug Administration. This interaction has provided a clear regul ...
